硼-硼配体-配体-同分异构体导致远距离 C-C 键和同时形成硼配体的证据
IF 3.3
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
报告了一种与 Py2BH(Py = 吡啶)型硼基供体配体的铑 PBP 型钳状配合物及其去质子化过程,该过程在形成新的 C-C 键的同时产生了硼烷基配合物。机理研究表明,两个去质子化的物种之间存在同分异构现象,从而产生了配体稳定的硼化物和配体稳定的硼烯基团。本文章由计算机程序翻译,如有差异,请以英文原文为准。
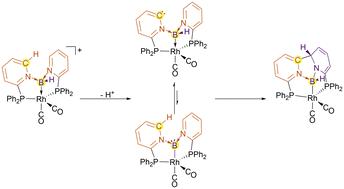
Evidence of boride–borylene ligand-tautomerism leading to a remote C–C-bond and concomitant boryl ligand formation†
The formation of a rhodium pincer-type complex with a boron-based donor ligand and its reactivity are reported. The starting complex contains a formal borylene moiety, stabilised by two pyridine substituents. Quantum chemical investigations indicate the possibility of deprotonation of the central donor group of the type py2BH in this complex. Efforts to isolate the resulting formal boride species, however, led to a boryl complex with concomitant formation of a new C–C-bond, accompanied by a loss of aromaticity. Mechanistic investigations indicate the presence of tautomerism between two deprotonated species, giving rise to a ligand-stabilised boride and a ligand-stabilised borylene motif.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


