石墨支撑的银、金和铜催化剂上的乙烯氢化过程中界面氢的作用
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
采用表面科学/微反应器相结合的方法,研究了碳支撑的银、金和铜纳米粒子催化剂在乙烯氢化过程中的界面效应。支撑催化剂的翻转频率(TOF)大大高于(无支撑)多晶金属箔,尤其是银。在高取向热解石墨(HOPG)和碳涂层网格上对相应金属进行火花烧蚀,可获得 3 纳米左右的纳米颗粒,非常适合通过 X 射线光电子能谱(XPS)、高分辨率(扫描)透射电子显微镜(HRTEM/STEM)和能量色散 X 射线能谱(EDX)进行表征。用扫描电子显微镜 (SEM)、EDX、电子反向散射衍射 (EBSD)、XPS 和低能离子散射 (LEIS) 表征的多晶金属箔作为无支撑参考。利用与超高真空兼容的流动微反应器和气相色谱法(GC),我们确定了模型催化剂在常压下进行乙烯氢化(最高温度可达 200 °C)时的催化性能。与纯金属箔相比,HOPG 支持的金属纳米颗粒不仅活性大幅提高,而且稳定性更高(失活速度更慢),反应顺序也不同。对活性最高的银催化剂进行了 DFT 计算,以确定反应物在单晶表面以及碳支撑和无支撑银纳米粒子上的吸附能。分子氢在所有无支撑的银表面上的吸附都非常弱,导致表面碳氢化合物 "中毒"。然而,当存在碳支持时,由于金属-碳三相边界附近的银-银距离发生了变化(而表面下的碳降低了氢键),银纳米粒子上的氢吸附强度平均增加了-0.5 eV。在铜粒子上,界面效应的计算结果比银粒子要弱一些。然后,在银催化剂上进行的 H2/D2 扰乱实验证实了碳支撑金属对氢活化的促进作用。因此,碳支撑效应可归因于金属-碳界面上氢可用性的改善,从而控制性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
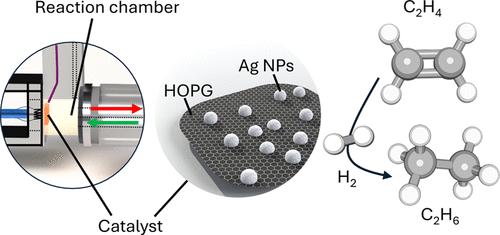
Role of Interfacial Hydrogen in Ethylene Hydrogenation on Graphite-Supported Ag, Au, and Cu Catalysts
A combined surface science/microreactor approach was applied to examine interface effects in ethylene hydrogenation on carbon-supported Ag, Au, and Cu nanoparticle catalysts. Turnover frequencies (TOFs) were substantially higher for supported catalysts than for (unsupported) polycrystalline metal foils, especially for Ag. Spark ablation of the corresponding metals on highly oriented pyrolytic graphite (HOPG) and carbon-coated grids yielded nanoparticles of around 3 nm size that were well-suited for characterization by X-ray photoelectron spectroscopy (XPS), high-resolution (scanning) transmission electron microscopy (HRTEM/STEM), and energy dispersive X-ray spectroscopy (EDX). Polycrystalline metal foils characterized by scanning electron microscopy (SEM), EDX, electron backscatter diffraction (EBSD), XPS, and low-energy ion scattering (LEIS) served as unsupported references. Employing a UHV-compatible flow microreactor and gas chromatography (GC) allowed us to determine the catalytic performance of the model catalysts in ethylene hydrogenation up to 200 °C under atmospheric pressure. Compared to the pure metal foils, the HOPG-supported metal nanoparticles exhibited not only strongly increased activity but also higher stability (slower deactivation) and differing reaction orders. For the most active Ag catalysts, DFT calculations were carried out to determine the adsorption energies of the reacting species on single-crystal surfaces as well as on carbon-supported and unsupported Ag nanoparticles. Adsorption of molecular hydrogen was very weak on all unsupported Ag surfaces, resulting in hydrocarbon-“poisoned” surfaces. However, when a carbon support was present, the adsorption strength of H2 on Ag nanoparticles increased on average by −0.5 eV, driven by changes in Ag–Ag distances near the metal–carbon three-phase boundary (whereas subsurface carbon lowers hydrogen bonding). On Cu particles, the interface effect was calculated to be somewhat weaker than for Ag particles. H2/D2 scrambling experiments on Ag catalysts then corroborated a facilitated hydrogen activation for carbon-supported metals. Thus, the carbon support effect is attributed to an improved hydrogen availability at the metal–carbon interface, controlling performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


