用于增强水吸附的复合金属有机框架的一锅合成:可行性与机理探索
IF 9.8
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
金属有机框架(MOF)通过大气集水(AWH)为解决全球水危机提供了一个前景广阔的解决方案。然而,它在各种湿度条件下的吸水性能限制了其实际应用。本研究采用一锅水热法合成了含有吸湿盐的复合 MOFs(CMOFs),以提高其吸水性,并研究了铝源和分散剂的影响。AlCl3和Al(NO3)3是高效的铝源,具有稳定的吸水性能,其中MOF-303具有优异的吸水性能,在20% RH条件下吸水容量为0.4 g-g-1。当使用 Al2(SO4)3 作为铝源时,MOF-303 的晶体结构会发生变化,比表面积明显降低,由于 SO42- 与 MOF-303 之间的结合能很强(-7.196 eV),因此在 20 % RH 条件下的吸水能力降至 0.2 g-g-1。通过一锅水热合成法成功地在 MOF 中加入了吸湿盐,MOF-303-LiOH、MOF-303-NaOH 和 MOF-303-Ca(OH)2 中盐的复合比分别为 0.1442、0.1732 和 0.1607 g-g-1。氯元素和钠元素的新化学状态表明,吸湿盐被 MOF 结构吸附/捕获。MOF-303-NaOH 在相对湿度为 30% 时的吸水能力稳定在 0.22 g-g-1,而在相对湿度为 95% 时的吸水能力则显著提高到 2.05 g-g-1。同时,CMOF 中盐类的比例可随分散剂的比例进行调整,循环吸附测试表明,AlFu-NaOH-2 可保持 1.62 g-g-1 的吸水能力,且无潮解现象。这项工作为合成具有优异水吸附性能的 CMOF 提供了一种新策略,有望促进 MOF 在 AWH 中的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
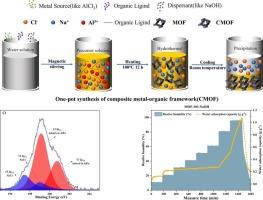
One-pot synthesis of composite metal-organic framework for enhanced water adsorption: Feasibility and mechanism exploration
Metal-organic framework (MOF) offers a promising solution to the global water crisis with atmospheric water harvesting (AWH). However, its practical application is limited by its water adsorption performance in various humidity conditions. In this work, composited MOFs (CMOFs) with hygroscopic salt were synthesized by a one-pot hydrothermal method to enhance the water uptake, and the effect of aluminum sources and dispersants was investigated. AlCl3 and Al(NO3)3 were efficient aluminum sources that showed stable water adsorption performance, which exhibited excellent water adsorption performance with a water adsorption capacity of 0.4 g·g−1 at 20 % RH for MOF-303. The crystal structure of MOF-303 will change and show a significantly low specific surface area as Al2(SO4)3 is used as the aluminum source, and the water adsorption capacity decreases to 0.2 g·g−1 at 20 % RH because of the strong binding energy (−7.196 eV) between SO42− and MOF-303. Hygroscopic salt was incorporated in MOF successfully via the one-pot hydrothermal synthesis method, the composite ratio of salt was 0.1442, 0.1732, and 0.1607 g·g−1 in MOF-303-LiOH, MOF-303-NaOH, and MOF-303-Ca(OH)2. The new chemical state of chlorine and sodium elements demonstrate that the hygroscopic salt was adsorbed/trapped by the MOF structure. MOF-303-NaOH showed a stable water adsorption capacity of 0.22 g·g−1 at 30 % RH and significantly enhanced water adsorption capacity of up to 2.05 g·g−1 at 95 % RH. Meanwhile, the ratio of salts in the CMOF can be adjusted with the proportion of dispersants, the cyclic adsorption test indicated that AlFu-NaOH-2 maintained the water adsorption capacity of 1.62 g·g−1 without deliquesce. This work provides a new strategy for synthesizing CMOFs with excellent water adsorption performance, which can potentially promote the application of MOF in AWH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


