长非编码 RNA VENTHEART 是心室心肌细胞规格和功能的必要条件
IF 4.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
理由:心脏表达的长非编码 RNA(lncRNA)对心肌细胞(CM)的分化和功能非常重要。目前已发现并鉴定了几种与早期心肌细胞谱系定向有关的 lncRNA,但对后期心肌细胞谱系定向和成熟过程中的 lncRNA 的研究仍然较少。方法与结果首先,我们对人多能干细胞衍生的心肌细胞(hPSC-CM)在分化后期2周、6周和12周进行了单细胞RNA-seq分析。加权相关网络分析确定了核心基因模块,包括一组在人类心脏中高度丰富且主要表达的lncRNA。一种lncRNA(我们称之为VENTHEART,VHRT)与心脏成熟和心室特异基因MYL2和MYH7共表达,在胎儿和成人心室中表达,但不在心房中表达。CRISPR 介导的 VHRT 基因缺失会导致 CM 肌节形成受损,并显著破坏心室 CM 基因程序。事实上,在 VHRT KO hPSC 衍生的心房 CM 中并未观察到类似的破坏,这表明 VHRT 只表现出心室肌细胞亚型特异性的影响。光学记录验证了 VHRT 的缺失会显著延长类心室而非类心房 CM 90% 复极时的动作电位持续时间(APD90)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
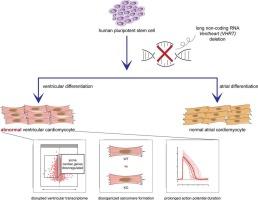
Long noncoding RNA VENTHEART is required for ventricular cardiomyocyte specification and function
Rationale
Cardiac-expressed long noncoding RNAs (lncRNAs) are important for cardiomyocyte (CM) differentiation and function. Several lncRNAs have been identified and characterized for early CM lineage commitment, however those in later CM lineage specification and maturation remain less well studied. Moreover, unique atrial / ventricular lncRNA expression has never been studied in detail.
Objectives
Here, we characterized a novel ventricular myocyte-restricted lncRNA, not expressed in atrial myocytes, and conserved only in primates.
Methods and results
First, we performed single cell RNA-seq on human pluripotent stem cell derived cardiomyocytes (hPSC-CM) at the late stages of 2, 6 and 12 weeks of differentiation. Weighted correlation network analysis identified core gene modules, including a set of lncRNAs highly abundant and predominantly expressed in the human heart. A lncRNA (we call VENTHEART, VHRT) co-expressed with cardiac maturation and ventricular-specific genes MYL2 and MYH7, and was expressed in fetal and adult human ventricles, but not atria. CRISPR-mediated deletion of the VHRT gene led to impaired CM sarcomere formation and significant disruption of the ventricular CM gene program. Indeed, a similar disruption was not observed in VHRT KO hPSC-derived atrial CM, suggesting that VHRT exhibits only ventricular myocyte subtype-specific effects. Optical recordings validated that loss of VHRT significantly prolonged action potential duration at 90 % repolarization (APD90) for ventricular-like, but not atrial-like, CMs.
Conclusion
This reports the first lncRNA that is exclusively required for proper ventricular, and not atrial, CM specification and function.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


