用于高效水解豆浆的灰口青霉α-半乳糖苷酶的纯化和表征
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2024-10-24
DOI:10.1016/j.bbrc.2024.150905
引用次数: 0
摘要
大豆的利用受到棉子糖低聚糖(RFO)存在的限制,这种低聚糖不能被人类消化,会引起胃肠道不适。本研究探讨了灰口青霉中的α-半乳糖苷酶水解豆浆中 RFO 的潜力。采用离子交换色谱法和原生聚丙烯酰胺凝胶电泳相结合的方法纯化了两种不同的 α-半乳糖苷酶,分别命名为 α-Gal1 和 α-Gal2。这两种酶都具有多聚蛋白的特征,并显示出相似的生化特性。在 pH 值为 4.5-5.0 和温度为 40-45 ℃ 的条件下观察到了最佳活性。值得注意的是,α-Gal1 具有很高的热稳定性,在 40 ℃ 下的半衰期为 16 h。α-gal乳糖苷酶对底物ρ-NP-αGal、o-NP-αGal、rD-棉子糖、d-水苏糖和mD-麦饭石糖具有不同的底物亲和力。α-Gal1的迈克尔斯-门顿常数(Km)分别为1.06、1.31、28.74、19.88和4.77毫摩尔/升,而α-Gal2的迈克尔斯-门顿常数(Km)分别为0.8、1.26、30.46、21.74和5.01毫摩尔/升。α-Gal1和α-Gal2均受到金属离子(Ag⁺、Cu2⁺、Fe2⁺和Hg2⁺)的强烈抑制,而受到d-麦芽糖的中度抑制。重要的是,这两种酶都能有效地水解 RFO,经过 6 小时的培养后,豆浆中的 d-水苏糖被完全消除。这些研究结果表明,这些α-半乳糖苷酶在工业豆浆生产中的应用前景广阔,有可能提高豆浆的营养价值,缓解消费者的胃肠道问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
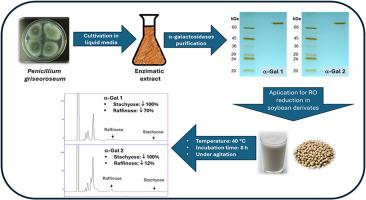
Purification and characterization of α-galactosidases from Penicillium griseoroseum for efficient soymilk hydrolysis
Soybean utilization is limited by the presence of raffinose oligosaccharides (RFO), which are not digested by humans and cause gastrointestinal discomfort. This study explores the potential of α-galactosidases from Penicillium griseoroseum for RFO hydrolysis in soymilk. Two distinct α-galactosidase enzymes, designated α-Gal1 and α-Gal2, were purified using a combination of ion-exchange chromatography and native polyacrylamide gel electrophoresis. Both enzymes exhibited characteristics of multimeric proteins and displayed similar biochemical properties. Optimal activity was observed at a pH range of 4.5–5.0 and a temperature range of 40–45 °C. Notably, α-Gal1 demonstrated high thermostability with a half-life of 16 h at 40 °C. The α-galactosidases displayed different substrate affinitiesfor the substrates ρ-NP-αGal, o-NP-αGal, rD-raffinose, d-stachyose, and mD-melibiose. The Michaelis-Menten constant (Km) values for α-Gal1 were 1.06, 1.31, 28.74, 19.88, and 4.77 mmol/L, respectively, while those for α-Gal2 were 0.8, 1.26, 30.46, 21.74 and 5.01 mmol/L, respectively. Both α-Gal1 and α-Gal2 were strongly inhibited by metal ions (Ag⁺, Cu2⁺, Fe2⁺, and Hg2⁺) and moderately inhibited by d-melibiose. Importantly, both enzymes efficiently hydrolyzed RFOs, achieving complete d-stachyose elimination from soymilk after a 6-h incubation. These findings propose the promising application of these α-galactosidases in industrial soymilk production, potentially enhancing its nutritional value and alleviating gastrointestinal issues in consumers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


