甘油醛-3-磷酸脱氢酶与 G-肌动蛋白的结合促进了反硝基化反应。
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在这项研究中,我们研究了甘油醛-3-磷酸脱氢酶(GAPDH)与肌动蛋白之间复合物的形成以及亚硝基基团在 GAPDH 与肌动蛋白之间转移的可能性。使用针对 GAPDH 或针对 beta-肌动蛋白的抗体进行免疫沉淀,从 HEK293T 细胞裂解物中分离出 GAPDH 与 beta-肌动蛋白的复合物。用 H2O2 或 NO 供体处理细胞不会影响复合物的形成。对纯化的 GAPDH 与肌肉α-肌动蛋白之间相互作用的研究表明,GAPDH 与球状(G-)肌动蛋白的相互作用优于与纤维肌动蛋白的相互作用,而且 GAPDH 的氧化/还原不会影响这种相互作用。在 G- 肌动蛋白存在的情况下,S-亚硝基化的 GAPDH(GAPDH-SNO)被部分重新激活,这伴随着 GAPDH 的变性和 G- 肌动蛋白的亚硫酸化。通过 MALDI-TOF MS 分析,确定了 G-actin 中硫化的半胱氨酸残基为 C 端 Cys374。根据亚硝硫醇的特性,我们推测肌动蛋白中的半胱氨酸亚硫酸是 S-亚硝基化半胱氨酸残基自发水解的产物。研究结果表明,肌动蛋白中的 Cys374 在与 GAPDH-SNO 培养过程中发生了 S-亚硝基化反应(转亚硝基化反应)。GAPDH-SNO 中的 NO 基团转移到肌动蛋白 C 端 Cys374 上,表明肌动蛋白与 GAPDH 相互作用后,其 C 端位于 GAPDH 的活性中心,靠近催化 Cys152。GAPDH-SNO 亚硝基化肌动蛋白的能力可能有助于肌动蛋白控制信号通路的氧化还原调节。本文章由计算机程序翻译,如有差异,请以英文原文为准。
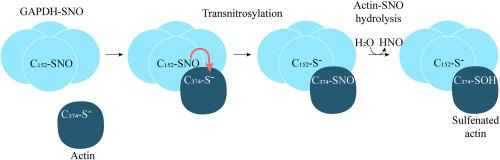
Binding of glyceraldehyde-3-phosphate dehydrogenase to G-actin promotes the transnitrosylation reaction
In this study, we investigated formation of the complex between glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and actin and the possibility of nitrosyl group transfer between GAPDH and actin. A complex of GAPDH with beta-actin was isolated from lysates of HEK293T cells using immunoprecipitation with antibodies against GAPDH or against beta-actin. The treatment of the cells with H2O2 or NO donor did not affect the formation of the complex. Investigation of the interaction between purified GAPDH and muscle alpha-actin showed that GAPDH interacts better with globular (G-) actin than with fibrillary actin, and oxidation/reduction of GAPDH does not affect this interaction. S-nitrosylated GAPDH (GAPDH-SNO) was partially reactivated in the presence of G-actin, which was accompanied by denitrosylation of GAPDH and sulfenation of G-actin. The sulfenated cysteine residue in G-actin was identified by MALDI-TOF MS analysis as C-terminal Cys374. Based on the properties of nitrosothiols, we assume that the cysteine-sulfenic acid in actin is a product of spontaneous hydrolysis of S-nitrosylated cysteine residue. The obtained results suggest that Cys374 in actin is S-nitrosylated during the incubation with GAPDH-SNO (transnitrosylation reaction). The transfer of the NO-group from GAPDH-SNO to the C-terminal Cys374 of actin suggests that upon interaction with GAPDH, the C-terminus of actin is located in the active center of GAPDH in the proximity to the catalytic Cys152. It is possible that the ability of GAPDH-SNO to nitrosylate actin contributes to the redox regulation of actin-controlled signaling pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


