(2R,6R)-羟基炔诺酮胺的简明合成。
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-11-15
Epub Date: 2024-11-01
DOI:10.1021/acs.joc.4c01502
引用次数: 0
摘要
从市场上可买到的材料开始,通过八个步骤完成了(2R,6R)-羟基炔草胺的简易合成。该合成的特点包括氯化铈增强的 Stork-Danheiser 反应、Corey-Bakshi-Shibata 反应对酮的不对称还原、标志性的 Overman 重排以及 RuCl3/NaIO4 对电子缺陷烯烃的面部选择性二羟基化。总产率为 7.3%,ee 为 94.5%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
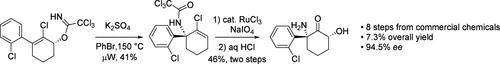
A Concise Synthesis of (2R,6R)-Hydroxynorketamine.
A concise synthesis of (2R,6R)-hydroxynorketamine was accomplished in eight steps, starting from commercially available materials. This synthesis features a cerium chloride-enhanced Stork-Danheiser reaction, an asymmetric reduction of ketone by the Corey-Bakshi-Shibata reaction, a signature Overman rearrangement, and a facial selective dihydroxylation of an electronically deficient olefin by RuCl3/NaIO4. The overall yield is 7.3% with 94.5% ee.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


