合成 securingine B 可实现光致发光材料的设计
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
一般来说,天然产物以其热力学上最稳定的形式存在。因此,导致热力学平衡的最后阶段反应条件往往有利于生产所需的天然产物。另一方面,反热力学天然产物的合成则面临更大的挑战,因为需要克服热力学偏差。我们利用两种天然产物之间三重能的差异,建立了有利于securingine B的光化学平衡。相反,secu'amamine D是在热力学平衡条件下从securingine B转化而来的。受这些观察结果的启发,我们在securinega 框架中引入了推拉系统,从而设计出了一种新型光开关平台。通过利用这种新型光开关支架,我们开发出了一种受securingine B启发的光致变色材料,并随后将其用作光致手性掺杂剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
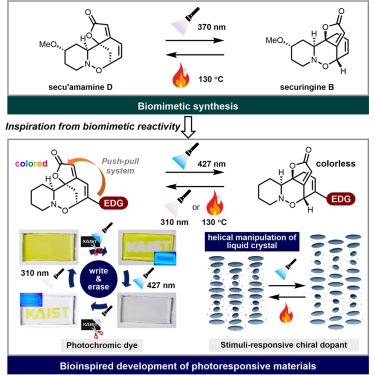

Synthesis of securingine B enables photoresponsive materials design
In general, natural products exist in their most thermodynamically stable form. Therefore, final stage-reaction conditions leading to thermodynamic equilibrium often facilitate the production of the desired natural products. On the other hand, syntheses of contra-thermodynamic natural products pose greater challenges, as the thermodynamic bias should be overcome. Herein, we present the synthesis of contra-thermodynamic securinega alkaloid securingine B, derived from the more thermodynamically stable isomer secu’amamine D. Harnessing the disparity in triplet energy between two natural products, we have established a photochemical equilibrium favoring securingine B. Conversely, secu’amamine D was reformed from securingine B under thermodynamic equilibrium conditions. Inspired by these observations, we devised a novel type of photoswitching platform by introducing a push-pull system to the securinega framework. By leveraging this new photoswitching scaffold, we have developed a securingine B-inspired photochromic material and, subsequently, exploited it as a photoresponsive chiral dopant.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


