将完全还原的多酮合成酶重新用于 2-甲基格尔伯特类脂质
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在自然界中,成千上万种具有生物活性的多酮苷是由一个名为多酮苷合成酶(PKS)的多功能 "流水线 "酶复合体家族组装而成的。自 20 世纪末以来,人们多次尝试解码、重新排列和 "重新编程 "PKS 组装线,以生成生物燃料和平台化学品等有价值的材料。在这里,我们对结核分枝杆菌(Mt)PKS12 的第一个模块(一种非正统的 "模块化迭代 "PKS)进行了改造和重新编程,以形成在工业中具有广泛应用的 2-甲基格尔伯特脂类。我们建立了一种重组表达和纯化这种改良模块(命名为 [M1*])的可靠方法,并证明了它催化形成几种 2-甲基格尔伯特类脂(C13-C21)的能力。此外,我们还研究并应用了邻近的β-酮酰合成酶(KS)的杂合硫酯酶活性,在一锅生物合成级联过程中释放与[M1*]结合的缩合产物。最后,我们以月桂酸为起点,通过将大肠杆菌脂肪酰基-CoA 合成酶 FadD 与 [M1*] 结合,生成了我们的主要目标化合物(2-甲基十四烷酸)。这项工作证明了[M1*]等工程化 PKS 模块的生物合成效用及其从廉价脂肪酸中提取有价值的格尔伯特类脂质的能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
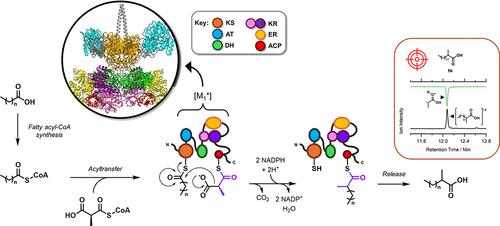
Repurposing a Fully Reducing Polyketide Synthase toward 2-Methyl Guerbet-like Lipids
In nature, thousands of diverse and bioactive polyketides are assembled by a family of multifunctional, “assembly line” enzyme complexes called polyketide synthases (PKS). Since the late 20th century, there have been several attempts to decode, rearrange, and “reprogram” the PKS assembly line to generate valuable materials such as biofuels and platform chemicals. Here, the first module from Mycobacterium tuberculosis (Mt) PKS12, an unorthodox, “modularly iterative” PKS, was modified and repurposed toward the formation of 2-methyl Guerbet lipids, which have wide applications in industry. We established a robust method for the recombinant expression and purification of this modified module (named [M1*]), and we demonstrated its ability to catalyze the formation of several 2-methyl Guerbet-like lipids (C13–C21). Furthermore, we studied and applied the promiscuous thioesterase activity of a neighboring β-ketoacyl synthase (KS) to release [M1*]-bound condensation products in a one-pot biosynthetic cascade. Finally, starting from lauric acid, we could generate our primary target compound (2-methyltetradecanoic acid) by coupling the Escherichia coli fatty acyl-CoA synthetase FadD to [M1*]. This work supports the biosynthetic utility of engineered PKS modules such as [M1*] and their ability to derive valuable Guerbet-like lipids from inexpensive fatty acids.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


