金属纳米结构在氨氧化成氧化亚氮的铈支撑催化剂中的作用
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
以 CeO2 为载体的锰(Mn)和铬(Cr)催化剂可将氨直接氧化为一氧化二氮(N2O),但由于缺乏合成-结构-性能关系,催化剂的合理设计受到阻碍。在此,我们生成了一个 CeO2 支持的锰和铬催化剂平台,系统地改变了从单原子到纳米颗粒的金属纳米结构以及载体的氧化还原特性,并通过先进的表征方法证实了这一点。CeO2 的表面还原性是控制 N2O 产率的一般描述因子。相反,结构敏感性具有金属特异性,以锰为基础的系统在单原子和纳米粒子形式下具有较高的 N2O 选择性,而以铬为基础的系统的选择性则取决于金属的分散性。原位紫外可见光(UV-vis)、稳态和瞬态动力学研究揭示了氧化还原活性 MnOx 与 CeO2 协同作用的能力,并按照 Mars-van Krevelen 机制增强了反应的氧气传输。这项研究从根本上揭示了催化剂各组分的作用和功能,为开发改良型 N2O 合成催化剂提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
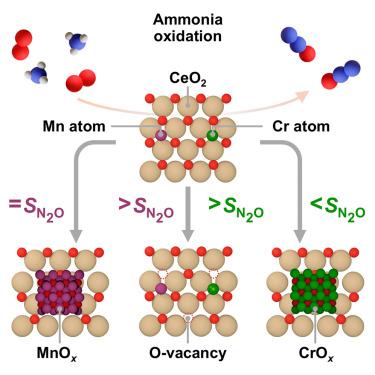
The role of metal nanostructure in ceria-supported catalysts for ammonia oxidation to nitrous oxide
Manganese (Mn) and chromium (Cr) catalysts supported on CeO2 enable direct ammonia oxidation to nitrous oxide, N2O, but the lack of synthesis-structure-performance relations hinders rational catalyst design. Herein, we generate a platform of CeO2-supported Mn and Cr catalysts, systematically varying the metal nanostructure from single atoms to nanoparticles, and the carrier redox properties, as confirmed by advanced characterization methods. Surface reducibility of CeO2 emerges as a general descriptor, controlling N2O productivity. Conversely, structure sensitivity is metal specific, with Mn-based systems achieving high N2O selectivity in single-atom and nanoparticle forms, while the selectivity of Cr-based systems is dependent on metal dispersion. In situ UV-visible (UV-vis), steady-state, and transient kinetic studies unveil the ability of redox-active MnOx to synergize with CeO2 and enhance oxygen transport for the reaction following a Mars-van Krevelen mechanism. This work provides fundamental insights into the role and function of each catalyst component and guidelines for the development of improved N2O synthesis catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


