手性 N-羟基烷基吡啶-2-亚基:铜催化不对称烯丙基烷基化的新型配体
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们开发出了一类源自吡啶的手性 N-杂环碳烯,即 N-羟烷基吡啶-2-亚基。这些配体利用吡啶-2-亚基核心的显著立体和电子特性以及氮原子上存在的手性羟烷基螯合臂,在铜催化的二烷基锌不对称烯丙基烷基化反应中,以高γ选择性(>98%)和对映选择性(高达 95% ee)将二烷基锌烷基化为各种烯丙基或二元磷酸盐,表现出了很高的性能。重要的是,催化剂负载量可降至 0.5 mol% 以下而不会降低催化剂效率,因此性能优于 N-羟基烷基咪唑啉-2-亚基同系物。此外,通过对所得到的对映体富集的跳过 1,4 二烯进行多功能后转化,还合成了各种相关的构筑基块,特别是 (+)-Phorbasin C 全合成过程中的一个关键中间体。氘化实验和计算研究为恶唑烷和相应的铜络合物的形成提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
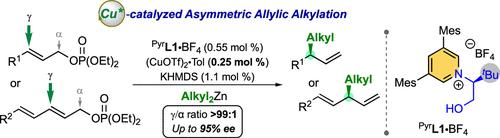
Chiral N-Hydroxyalkyl Pyrid-2-Ylidenes: A New Class of Ligands for Copper-Catalyzed Asymmetric Allylic Alkylation
A class of chiral N-heterocyclic carbenes derived from pyridine, namely N-hydroxyalkyl pyrid-2-ylidenes, was developed. Capitalizing the remarkable steric and electronic features of the pyrid-2-ylidene core with the presence of a chiral hydroxyalkyl-chelating arm on the nitrogen atom, these ligands demonstrated high performance in copper-catalyzed asymmetric allylic alkylation of dialkylzincs to various allylic or dienic phosphates with high γ-selectivity (>98%) and enantioselectivity (up to 95% ee). Importantly, the catalyst loading can be decreased to below 0.5 mol% without any loss of catalyst efficiency, thus outperforming N-hydroxyalkyl imidazoline-2-ylidene congeners. Moreover, thanks to the versatile post-transformation of the resulting enantioenriched skipped 1,4-dienes, various relevant building blocks were synthesized, notably a key intermediate in the total synthesis of (+)-Phorbasin C. Furthermore, by involving a transient oxazolidine, which acts as a masked carbene before the insertion of the metal center, a well-defined but air-sensitive N-hydroxyalkyl pyrid-2-ylidene copper(I) chloride complex was isolated. Deuteration experiments and computational studies provided valuable insights into the formation of the oxazolidine and the corresponding copper complex.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


