甲醇和乙醇电氧化起始电位和氧化电位的机器学习预测:综合分析与实验验证
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
电化学反应的起始电位和氧化电位是评估催化能效的关键,其应用横跨各个领域,包括可持续能源发电。然而,预测这些电位是一项复杂而未知的挑战。在本研究中,我们提出了一种开创性的方法,为甲醇和乙醇氧化相关电化学反应的起始电位和氧化电位开发预测模型。我们设计了一个从数据收集、信息提取到预处理的综合管道,并评估了不同回归模型的性能:线性模型、随机森林模型和 XGBoost 模型。在氧化潜能预测方面,RMSE 为 0.169,R2 值为 0.814。同样,在起始电位预测方面,模型的 RMSE 为 0.185,R2 值为 0.839。我们使用特征重要性和 SHAP 值对模型进行了进一步评估,从而加深了我们对其预测机制的理解,并提供了对特征的更多理解。此外,我们还进行了实验验证,将预测结果与在化学实验室进行的甲醇和乙醇氧化实验的实际结果进行了比较。验证过程包括使用铂、金、泡沫镍、钢和 RuO2/FTO 电极。令人鼓舞的是,实验验证结果令人鼓舞,起始电位的均方根误差为 0.0967,氧化电位的均方根误差为 0.0234。本文章由计算机程序翻译,如有差异,请以英文原文为准。
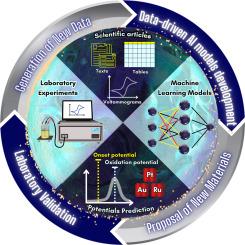
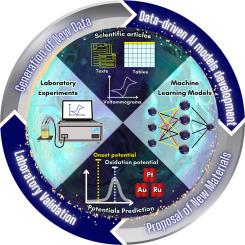
Machine learning predictions of onset and oxidation potentials for methanol and ethanol electrooxidation: Comprehensive analysis and experimental validation
The onset and oxidation potentials of electrochemical reactions are pivotal in assessing catalytic energy efficiency, spanning applications across various domains, including sustainable energy generation. However, predicting these potentials presents a complex and uncharted challenge. In this study, we present a pioneering approach to developing predictive models for the onset and oxidation potentials within electrochemical reactions linked to the oxidation of methanol and ethanol. We have devised a comprehensive pipeline from Data Collection, Information Extraction, and Preprocessing and assessed the performance of different regression models: Linear, Random Forest, and XGBoost. For the oxidation potential prediction, an RMSE of 0.169 and an R value of 0.814 were achieved. Similarly, for the onset potential prediction, the model yielded an RMSE of 0.185 and an R value of 0.839. The models were further evaluated using feature importance and SHAP values, enhancing our understanding of their predictive mechanisms and providing more comprehension of the features. Additionally, we conducted experimental validations by comparing the predicted outcomes to actual results obtained from methanol and ethanol oxidation experiments carried out in a chemical laboratory. This validation process included the utilization of platinum, gold, nickel foam, steel and RuO/FTO electrodes. Encouragingly, the experimental validation yielded promising findings, exhibiting an RMSE of 0.0967 for the onset potential and an RMSE of 0.0234 for the oxidation potential.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


