细胞苷元的简易合成方法以及曲霉香豆素 A 和 fusarimarin C 的首次全合成
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究展示了首次全合成具有生物活性的异香豆素天仙子香豆素 A、扶桑香豆素 C 和细胞苷元的新颖、高效路线。这种方法的特点是,在 LiBr-LiTMP 复合物存在的情况下,利用二甲氧基丁二酸乙酯和乙醇酸乙酯的高难度交叉酯偶联,开创性地一步合成异香豆素。该工艺利用溴离子诱导的聚合作用来促进单锅串联反应序列:侧向石碳酸化、酰化、烯醇化和内酯化。这种创新方法能高效地直接生成异香豆素分子。随后的转化过程包括选择性 BBr3 介导的去甲基化、氧化和 Wittig 反应。所展示的简化合成路线包括:两步合成细胞苷元,四步合成曲霉香豆素 A 和扶桑香豆素 C,总产率分别为 37%、27% 和 27%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
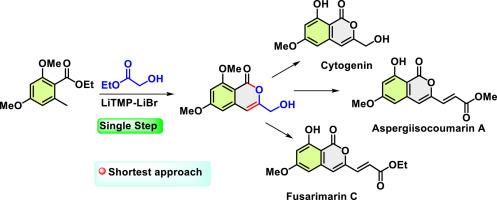
A short approach to cytogenin and first total synthesis of aspergisocoumarin A and fusarimarin C
A novel, efficient route to the first total synthesis of the bioactive isocoumarins aspergisocoumarin A, fusarimarin C and cytogenin is demonstrated. This approach features a groundbreaking single-step synthesis of isocoumarins utilizing a challenging cross-ester coupling of ethyl dimethoxy orsellinate and ethyl glycolate in the presence of a LiBr-LiTMP complex. The process leverages bromide ion-induced aggregation to facilitate a one-pot tandem reaction sequence: lateral lithiation, acylation, enolization, and lactonization. This innovative method directly produces the isocoumarin moiety with high efficiency. Subsequent transformations include selective BBr3-mediated demethylation, oxidation, and Wittig reactions. The streamlined synthetic routes demonstrated: a two-step pathway to cytogenin and a four-step sequence to aspergisocoumarin A and fusarimarin C, achieving overall yields of 37 %, 27 %, and 27 % respectively.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


