PPh3 促进以三氯乙酸乙酯和芳基醛为原料立体特异性合成 α-氯丙烯酸乙酯
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在温和的条件下,PPh3 可促进芳基醛与三氯乙酸乙酯之间的偶联反应,从而立体定向地生成 (Z)- α-氯丙烯酸乙酯。这种立体特异性偶联反应只有在芳基醛衍生物具有抽电子取代基时才会发生。另一方面,当芳香环中存在一个电子奉献基团时,即使在低温条件下,芳基二氯甲基衍生物也是唯一的产物。根据 13C NMR 和晶体-X 射线分析中的耦合常数 2JCH 和 3JCH,确定了耦合反应的立体特异性。根据这些数据,我们认为这些化合物是通过 PPh3 和三氯乙酸乙酯反应最初形成的两个中间体形成的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
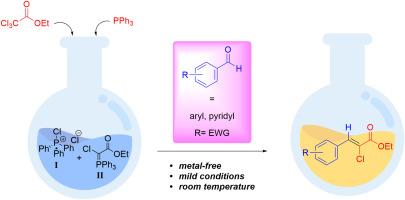
PPh3 promoted stereospecific synthesis of ethyl α-chloroacrylates from ethyl trichloroacetate and arylaldehydes
A coupling reaction between arylaldehydes and ethyl trichloroacetate is facilitated by PPh3 under mild conditions to stereospecifically produce (Z)-ethyl α-chloroacrylates. This stereospecific coupling reaction only occurs when the arylaldehyde derivative has an electron-withdrawing substituent. On the other hand, when an electron-donating group is present in the aromatic ring, the aryldichloromethyl derivative is formed as the only product, even at low temperature. The stereospecificity in the coupling reaction was established based on the coupling constants 2JCH and 3JCH in the 13C NMR and crystal-X-ray analysis. From this data we propose that the formation of these compounds occurs through two intermediates initially formed by the reaction between the PPh3 and ethyl trichloroacetate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


