以牛磺酸为绿色生物有机催化剂,在水介导下可持续地一步合成 2-羟基-5-氧代-5,6,7,8-四氢-4H-色烯和香豆素衍生物
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究报告了在回流条件下合成 4-芳基-2-羟基-7,7-二甲基-5,6,7,8-四氢-4H-苯并吡喃-5-酮 (3a-j) 和 3-取代香豆素衍生物 (3′a-f) 的两种牛磺酸催化的水介导方法。这些方法包括使用取代的芳香醛、丙二酸二乙酯和二甲基酮作为试剂,通过克诺文那格尔-迈克尔级联反应可持续合成 2-羟基-四氢-4H-苯并吡喃衍生物;以及使用取代的水杨醛与丙二酸二乙酯或 3-氧代丁酸乙酯,通过克诺文那格尔-酯化反应高效合成香豆素衍生物。这些策略强调环境的可持续性,并设计为单锅工艺,确保简单方便。这些方法产率高,成本效益高,大大缩短了反应时间,提高了步骤效率,并在多克水平上取得了成功。值得注意的是,牛磺酸催化剂具有卓越的可重复使用性,其活性可维持长达五个循环,且无明显降解。通过过滤,催化剂很容易回收,而且整个程序简化,不需要额外的纯化步骤。这些特性共同促进了绿色化学的发展,最大限度地减少了废物和能源消耗,从而为合成这些重要的有机化合物提供了一种实用、高效的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
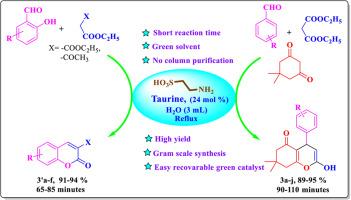
Sustainable aqueous-mediated one-pot synthesis of 2-hydroxy-5-oxo-5,6,7,8-tetrahydro-4H-chromene and coumarin derivatives using taurine as the green bioorganic catalyst
In this study, two taurine-catalyzed, aqueous-mediated methodologies were reported for the synthesis of 4-aryl-2-hydroxy-7,7-dimethyl-5,6,7,8-tetrahydro-4H-chromen-5-one (3a-j) and 3-substituted coumarin derivatives (3′a-f) under reflux conditions. These methodologies entail the use of substituted aromatic aldehydes, diethyl malonate, and dimedone as reagents for the sustainable synthesis of 2-hydroxy-tetrahydro-4H-chromene derivatives via the Knoevenagel-Michael cascade, as well as the use of substituted salicylaldehyde with diethyl malonate or ethyl 3-oxobutanoate for the efficient synthesis of coumarin derivatives via the Knoevenagel-transesterification reaction. These strategies emphasize environmental sustainability and are designed as one-pot processes, ensuring simplicity and convenience. The methods deliver high yields and are cost-effective, significantly reducing reaction times, enhancing step efficiency, and are successful at the multigram level. Notably, the taurine catalyst demonstrates exceptional reusability, maintaining its activity for up to five cycles without notable degradation. The catalyst is easily recoverable through filtration, and the overall procedure is streamlined, requiring no additional purification steps. These attributes collectively contribute to the green chemistry paradigm by minimizing waste and energy consumption, thus offering a practical and efficient approach to the synthesis of these important organic compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


