研究 44-甲基甘比龙与高碘酸盐的反应性:结构重定向、溶剂不稳定性和呋喃类似物的形成
IF 2.6
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
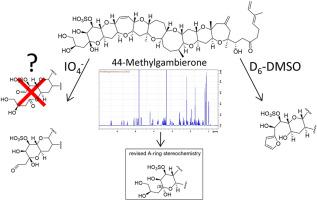
冈比埃隆是由冈比埃迪斯克斯属、酷利亚属和福尤亚属的底栖甲藻产生的硫酸化聚醚。虽然与雪卡毒素类似物相比,冈比埃隆的相对毒性较低,但有人建议将冈比埃隆用作环境监测计划的标记化合物,以确定海洋水域中是否存在冈比狄斯藻。据报道,已公布的甘比耶酮及其类似物(包括 44-甲基甘比耶酮(44-MeGAM))的结构具有 1,2- 和 4,5- 顺式二醇,而只有 1,2- 二醇单元被证明会发生高碘酸盐氧化。对之前报道的 44-MeGAM 在 CD3OD 中的核磁共振数据进行深入分析后发现,44-MeGAM 和其他甘比二酮的 C-4 立体化学结构被错误地指定,4-CH2-CHOH-CH2OH 和 OH 基团分别呈赤道和轴向,而不是之前报道的反向。对现有 44-MeGAM NMR 数据的重新研究还表明,它的 C-12 和 C-13 定义(以及其他甘珀酮的定义)应该颠倒过来。为了更好地了解甘珀酸酮的 C-4 立体化学特征和高碘酸反应特征(C-4 是一个可表聚的半缩醛碳),我们在 D6-DMSO 中获取了更多核磁共振数据。意外的是,我们观察到 44-MeGAM 逐渐转化为长期稳定的环-A 呋喃类似物。随后进行的一系列微观稳定性试验确定了影响 44-MeGAM 溶液稳定性的几种溶剂,在今后的研究中,在对甘草酮进行分离、处理、储存和生物测定评估时应考虑到这些发现。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigation of 44-methylgambierone reactivity with periodate: Structural reassignment, solvent instability and formation of a furanoid analogue
Gambierones are sulfated polyethers produced by benthic dinoflagellates in the genera Gambierdiscus, Coolia and Fukuyoa. While relative toxicity data for gambierones suggests they are low compared with ciguatoxin analogues, gambierones have been suggested for use as marker compounds for environmental monitoring programs for the presence of Gambierdiscus in marine waters. The published structure of gambierone and analogues of it, including 44-methylgambierone (44-MeGAM), have been reported to possess 1,2- and 4,5-cis diols, while only the 1,2- diol unit has been shown to undergo periodate oxidation. An in-depth analysis of previously reported NMR data for 44-MeGAM in CD3OD showed that the C-4 stereochemistry of 44-MeGAM and other gamberiones was mis-assigned, that the 4-CH2-CHOH-CH2OH and OH groups are equatorially and axially oriented, respectively, rather than vice versa as previously reported. This re-examination of existing 44-MeGAM NMR data also showed that its C-12 and C-13 assignments (and those for other gambierones) should be reversed. In an effort to better understand the C-4 stereochemical and periodate reaction characteristics of gambierones (C-4 is an epimerizable hemiacetal carbon), additional NMR data was acquired in D6-DMSO. Unexpectedly, progressive conversion of 44-MeGAM to a long-term stable ring-A furanoid analogue was observed. A subsequent series of microscale stability trials identified several solvents that affected the solution-stability of 44-MeGAM, and these findings should be taken into consideration during isolation, handling, storage and bioassay evaluations of gambierones in future studies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


