来自成纤维细胞生长因子 1 预处理脂肪来源干细胞的工程外泌体通过激活自噬促进缺血皮瓣存活
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
背景缺血皮瓣的恢复是临床上的一个主要问题。本研究的目的是评估由FGF1预处理脂肪干细胞(FEXO)衍生的工程外泌体对缺血性皮瓣的影响。方法 本研究招募了6名褥疮4期并接受皮瓣手术的患者,以筛选FGF家族中缺血性皮瓣的潜在靶点。将 FGF1 与脂肪干细胞共培养,并采用超速离心法提取 FEXO。利用转录组测序分析确定了 FEXO 中最有效的 microRNA。我们的研究建立了动物皮瓣模型,以验证 FEXO 的效果。结果FGF1被认为是缺血性皮瓣的治疗和诊断靶点,但在挽救皮瓣方面仍存在不足。FEXO通过抑制氧化应激,通过增强自噬通量缓解细胞凋亡和热凋亡,从而明显提高了RPSFs和内皮细胞的活力。此外,FEXO 还能抑制过度激活的炎症反应。转录组测序分析表明,miR-183-5p在FEXO中显著升高,抑制miR-183-5p会导致皮瓣自噬的保护作用受损。外泌体miR-183-5p通过Pi3k/Akt/mTOR通路靶向GPR137,明显增强了细胞活力,抑制了氧化应激,缓解了内皮细胞的凋亡和热凋亡,表明GPR137也可能是缺血性皮瓣的治疗靶点。结论FEXO通过miR-183-5p/GPR137/Pi3k/Akt/mTOR轴显著提高了缺血性皮瓣的存活率,这将是挽救缺血性皮瓣的一种有前途的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
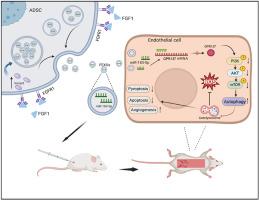
Engineering exosomes from fibroblast growth factor 1 pre-conditioned adipose-derived stem cells promote ischemic skin flaps survival by activating autophagy
Background
The recovery of ischemic skin flaps is a major concern in clinical settings. The purpose of this study is to evaluate the effects of engineered exosomes derived from FGF1 pre-conditioned adipose-derived stem cells (FEXO) on ischemic skin flaps.
Method
6 patients who suffered from pressure ulcer at stage 4 and underwent skin flaps surgery were recruited in this study to screen the potential targets of ischemic skin flaps in FGF family. FGF1 was co-incubated with adipose stem cells, and ultracentrifugation was applied to extract FEXO. Transcriptome sequencing analysis was used to determine the most effective microRNA in FEXO. Animal skin flaps models were established in our study to verify the effects of FEXO. Immunofluorescence (IF), western blotting (WB) and other molecular strategy were used to evaluate the effects and mechanism of FEXO.
Results
FGF1 was expected to be the therapeutic and diagnostic target of ischemic skin flaps, but there is still some deficiency in rescuing skin flaps. FEXO significantly improved the viability of RPSFs and endothelial cells by inhibiting oxidative stress and alleviating apoptosis and pyroptosis through augmenting autophagy flux. In addition, FEXO inhibited the over-activated inflammation responses. Transcriptome sequencing analysis showed that miR-183-5p was significantly elevated in FEXO, and inhibiting miR-183-5p resulted in impaired protective effects of autophagy in skin flaps. The exosomal miR-183-5p markedly enhanced cell viability, inhibited oxidative stress and alleviated apoptosis and pyroptosis in endothelial cells by targeting GPR137 through Pi3k/Akt/mTOR pathway, indicating that GPR137 could also be a therapeutic target of ischemic skin flap. It was also notabale that FGF1 increased the number of exosomes by upregulating VAMP3, which may be a promising strategy for clinical translation.
Conclusion
FEXO markedly improved the survivial rate of ischemic skin flaps through miR-183-5p/GPR137/Pi3k/Akt/mTOR axis, which would be a promising strategy to rescue ischemic skin flaps.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


