用于溴硝基苯选择性氢化的铜单原子装饰铱纳米粒子
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
金属间的相互作用是改变金属催化剂催化作用的重要因素,揭示金属间相互作用与催化性能之间的关系是非常有必要的。本研究制备了三种类型的 Ir-Cu 催化剂,包括 Cu 单原子修饰的 Ir 纳米颗粒和 Ir-Cu 合金催化剂,以观察 Cu 对 Ir 的修饰作用。在相对较高的温度处理下,可以用 Cu 单原子和 Ir 纳米粒子制备出 Ir-Cu 合金催化剂。在 Cu/C-Ir 催化剂上,溴代苯胺(BAN)的选择性可达 99.9%,溴代硝基苯(BNB)的转化率为 100%,高于 Ir/C 催化剂(94.3%)和 Ir-Cu/C 合金催化剂(98.5%)。Cu/C-Ir 催化剂的翻转频率 (TOF) 明显低于 Ir-Cu/C 合金催化剂,但其分散度高于 Ir-Cu/C 合金催化剂。从 Cu 单原子到 Ir 纳米粒子的电子转移不仅削弱了氢吸附能力,而且减少了可将氢分子分裂成氢原子的活性位点数量,导致 BNB 选择性加氢的催化活性降低。未合金化的铜单质与合金化的铜单质在铱纳米颗粒上产生的相互作用不同,这可能是铜单质修饰的铱纳米颗粒与铱铜合金催化性能不同的原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
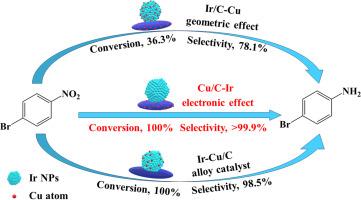
Copper single atoms decorated iridium nanoparticles for the selective hydrogenation of bromonitrobenzene
The interaction between metals is an important factor to modify the catalytic roles of metallic catalysts, and it is highly desirable to reveal the relationship between metal-metal interaction and catalytic performance. Three types of Ir-Cu catalysts including Cu single atoms modified Ir nanoparticles and Ir-Cu alloy catalysts are prepared to observe the modifying role of Cu on Ir. Under relatively high temperature treatment, Ir-Cu alloy can be produced from Cu single atoms and Ir nanoparticles. The selectivity to bromoaniline (BAN) over Cu/C-Ir catalyst can be up to 99.9 % at the 100 % conversion of bromonitrobenzene (BNB), which is higher than that over Ir/C (94.3 %) and Ir-Cu/C alloy catalyst (98.5 %). The turnover frequencies (TOFs) of Cu/C-Ir catalysts are evidently lower than that of the Ir-Cu/C alloy catalyst, though their dispersions are higher than that of Ir-Cu/C alloy catalyst. The electronic transfer from Cu single atoms to Ir nanoparticles not only weakens the hydrogen adsorption ability but reduces the number of active sites available for splitting hydrogen molecules into hydrogen atoms, leading to a decrease in the catalytic activity for the selective hydrogenation of BNB. The interaction exerted by unalloyed Cu single atoms on Ir nanoparticles differs from that of alloyed Cu on Ir, which may be the possible reason for the different catalytic performance between Cu single atoms modified Ir nanoparticles and Ir-Cu alloy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


