低温 NH3-SCR 反应中 MnOx-CeO2/Ti 轴高炉矿渣催化剂的 SO2 中毒机理探析
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
硫中毒是 NH3-SCR 催化剂面临的一个棘手难题。本课题通过催化活性评价、BET、XRD、XPS、NH3-TPD、H2-TPR 和原位 DRIFTS 分析,阐述了二氧化硫导致含 MnOx-CeO2/Ti 高炉渣催化剂失活的内在原因。结果表明,MnOx-CeO2/Ti-含高炉矿渣催化剂遵循 E-R 反应机理,在 175 ℃ 下可实现 100 % 的 NO 转化,但其抗硫性不理想,且随着 SO2 浓度的增加,失活程度越来越严重。SO2 与 NH3 反应生成 (NH4)2SO4 和 NH4HSO4 沉淀,堵塞了催化剂孔隙并覆盖了活性位点。SO2 还与 MnOx-CeO2 活性成分发生作用,生成 MnSO4 和 Ce2(SO4)3,从而限制了 Mn4+/Mnn+ 和 Ce3+/Ce4+ 的电子转移。此外,新形成的含硫酸性位点还与原来的活性位点竞争吸附 NH3,从而阻碍了 SCR 反应。物理净化无法实现 SO2 中毒催化剂的再生,而热处理则是一种有效的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
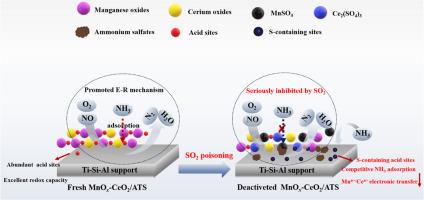
Insight into SO2 poisoning mechanism of MnOx-CeO2/Ti-bearing blast furnace slag catalyst for low temperature NH3-SCR reaction
Sulfur poisoning is an intractable challenge for NH3-SCR catalyst. In this issue, the immanent reasons for MnOx-CeO2/Ti-bearing blast furnace slag catalyst deactivation resulting from SO2 were elaborated by catalytic activity evaluation, BET, XRD, XPS, NH3-TPD, H2-TPR and in-situ DRIFTS analysis. Results showed that MnOx-CeO2/Ti-bearing blast furnace slag catalyst followed E-R reaction mechanism and performed 100 % NO conversion at 175 °C, whereas its sulfur resistance was unsatisfactory and the deactivation degree became more severe with SO2 concentration increasing. SO2 reacted with NH3 to generate (NH4)2SO4 and NH4HSO4 deposits, blocking catalyst pores and covering active sites. SO2 also interacted with MnOx-CeO2 active components to form MnSO4 and Ce2(SO4)3, which restricted the electron transfers of Mn4+/Mnn+ and Ce3+/Ce4+. Besides, the newly formed sulfur-containing acidic sites also competed with the original active sites for NH3 adsorption, thereby hindering the SCR reaction. Physical purging could not realize regeneration of SO2-poisoning catalyst, but hyperthermic treatment was an efficient solution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


