从地衣中提取高质量元基因组 DNA
IF 1.7
4区 生物学
Q4 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
地衣是遍布温带陆地森林的复合生物,与工业空气污染有特定的物种关联。地衣样本的元基因组分析需要强大的核酸提取方法,由于地衣具有保护性皮层、多糖含量高以及内部微生物群的多样性,这一过程极具挑战性。我们的方法包括通过石榴石打珠进行物理裂解、使用十二烷基硫酸钠缓冲液进行化学裂解、苯酚:氯仿:异戊醇提取以及乙醇沉淀。该方法在来自两个不同物种的三个不同地衣样本上进行了测试,获得了适合测序和 PCR 扩增的元基因组 DNA。该方法使用普通的实验室设备和试剂,无需使用液氮,解决了从地衣中提取 DNA 的相关问题。本文介绍了一种从干燥和保存的地衣标本中获得高质量遗传物质的成本效益高且容易获得的 DNA 提取方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
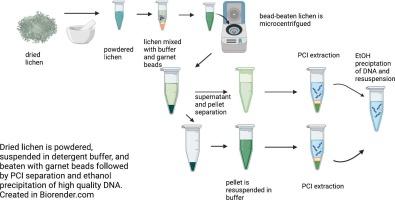
Extraction of high-quality metagenomic DNA from the lichens Flavoparmelia caperata and Peltigera membranacea
Lichens are composite organisms found throughout temperate terrestrial forests, with species-specific associations with industrial air pollution. Metagenomic analysis of lichen samples requires robust nucleic acid extraction methodology, a process that is challenging due to the protective cortex layers, high polysaccharide content, and the vast diversity of the internal microbiome. Our method includes physical lysis through garnet bead beating, chemical lysis using a sodium dodecyl sulfate buffer, phenol:chloroform:isoamyl alcohol extraction, and ethanol precipitation. The method was tested on three different lichen samples from two distinct species and yielded metagenomic DNA suitable for sequencing and PCR amplification. This procedure addresses the issues associated with DNA extraction from lichen using common laboratory equipment and reagents without the utilization of liquid nitrogen. This paper presents a cost-effective and accessible DNA extraction method for obtaining high-quality genetic material from dried and preserved lichen specimens.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of microbiological methods
生物-生化研究方法
CiteScore
4.30
自引率
4.50%
发文量
151
审稿时长
29 days
期刊介绍:
The Journal of Microbiological Methods publishes scholarly and original articles, notes and review articles. These articles must include novel and/or state-of-the-art methods, or significant improvements to existing methods. Novel and innovative applications of current methods that are validated and useful will also be published. JMM strives for scholarship, innovation and excellence. This demands scientific rigour, the best available methods and technologies, correctly replicated experiments/tests, the inclusion of proper controls, calibrations, and the correct statistical analysis. The presentation of the data must support the interpretation of the method/approach.
All aspects of microbiology are covered, except virology. These include agricultural microbiology, applied and environmental microbiology, bioassays, bioinformatics, biotechnology, biochemical microbiology, clinical microbiology, diagnostics, food monitoring and quality control microbiology, microbial genetics and genomics, geomicrobiology, microbiome methods regardless of habitat, high through-put sequencing methods and analysis, microbial pathogenesis and host responses, metabolomics, metagenomics, metaproteomics, microbial ecology and diversity, microbial physiology, microbial ultra-structure, microscopic and imaging methods, molecular microbiology, mycology, novel mathematical microbiology and modelling, parasitology, plant-microbe interactions, protein markers/profiles, proteomics, pyrosequencing, public health microbiology, radioisotopes applied to microbiology, robotics applied to microbiological methods,rumen microbiology, microbiological methods for space missions and extreme environments, sampling methods and samplers, soil and sediment microbiology, transcriptomics, veterinary microbiology, sero-diagnostics and typing/identification.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


