水热法提高 ZnCo2S4@ 金属有机框架复合电极的电容保持率
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
金属有机框架衍生材料因其比表面积、多孔性和优异的氧化还原行为而成为电化学超级电容器的理想电极。本研究通过固态研磨和水热处理合成了 ZnCo2S4 和 ZnCo2S4@ZIF-67 复合材料,用于超级电容器。使用 XRD、XPS、傅立叶变换红外光谱、BET、FE-SEM 和 HR-TEM 进行研究,以验证其形态、表面和结构特征。高导电性 ZnCo2S4 纳米结构材料与 MOF 表面插层,增强了电子传输。在使用 1 M KOH 电解质时,电化学相快速波动所涉及的大量活性位点可能是造成这种现象的原因。ZnCo2S4 和 ZnCo2S4@ZIF-67 复合材料用于制造工作电极,而 1 M KOH 电解液则用于制造超级电容器。通过采用三电极设计,创建的复合电极在 1 A/g 时的比电容分别为 245F/g 和 447.14F/g,从而提高了循环保持率。在制造 ZnCo2S4@ZIF-67/1M KOH/SSC 时使用了两种电极配置,在 1 A/g 时的比电容为 151.42F/g,在 7 A g-1 循环 7000 次时的电容保持率为 85.2%,在 642.85 W kg-1 功率密度下的能量密度为 18.93 Wh kg-1。因此,所制造的复合电极可通过双电极配置系统应用于电化学对称超级电容器。本文章由计算机程序翻译,如有差异,请以英文原文为准。
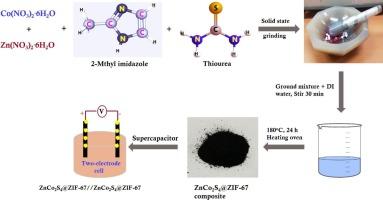
Enhancement of capacitance retention of ZnCo2S4@Metal organic framework composite electrodes by hydrothermal process
Metal organic framework-derived materials are promising electrodes for electrochemical supercapacitors due to their surface area, porosity, and excellent redox behaviors. In the present study the fabrication of ZnCo2S4 and ZnCo2S4@ZIF-67 composites synthesized by solid-state grinding and hydrothermal processing for supercapacitor utilization. Studies using XRD, XPS, FTIR, BET, FE-SEM, and HR-TEM are employed to validate the morphological, surface, and structural characteristics. Highly conductive ZnCo2S4 nanostructured materials are intercalated with MOF surfaces to enhance electron transport. The high number of active sites involved in the rapid electrochemical phase fluctuation using 1 M KOH electrolyte may be the cause of this. ZnCo2S4 and ZnCo2S4@ZIF-67 composites are used to create the working electrode, while a 1 M KOH electrolyte is used for the supercapacitor. By employing a three-electrode design, the created composite electrodes improve cyclic retention with specific capacitances of 245 and 447.14F/g at 1 A/g, respectively. Two electrode configurations are used to build ZnCo2S4@ZIF-67/1M KOH/SSC, which produced results of 151.42F/g at 1 A/g, 85.2 % capacitance retention at 7 A g−1 of 7000 cycles, and 18.93 Wh kg−1 energy density at 642.85 W kg−1 power density. Thus, the fabricated composite electrodes may find application in electrochemical symmetric supercapacitor via two electrode configuration systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


