原位形成亚纳米钴颗粒与铂纳米晶,用于高性能氧还原反应电催化剂
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
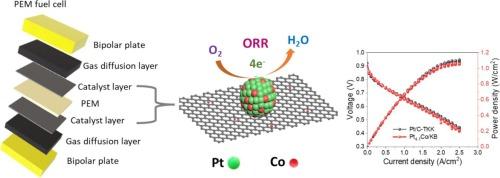
迄今为止,铂(Pt)是质子交换膜燃料电池(PEMFC)氧还原反应(ORR)中最活跃的电催化剂。提高性能和减少使用昂贵的铂对 PEMFC 的广泛应用具有重要意义。本研究通过一种简单的多元醇方法,将原位合成的铂纳米晶体与亚纳米尺寸的钴(Co)颗粒(≤ 0.3 nm)固定在黑碳(KB)载体上,作为一种高活性 ORR 电催化剂。合成的 Pt4.1Co/KB 催化剂具有 0.925 V 的正半波电位,在 0.1 M HClO4 溶液中 0.9 V 的电位下,其质量活性是无钴 Pt/KB 催化剂的 1.8 倍,且在 30,000 次电位循环后 ORR 性能衰减不明显。Pt4.1Co/KB 的优异电催化性能在实际的 H2/air 燃料电池中也得到了证实,其最大峰值功率密度为 1.08 W/cm2,与标准的 Pt/C-TKK (47 %) 催化剂相当。Pt4.1Co/KB ORR 性能的提高归功于亚纳米级 Co 粒子的加入,它们协同提高了催化剂的活性和稳定性。利用周期密度泛函理论进行的计算研究还表明,超细 Co 纳米粒子的加入使铂对状态密度的贡献向高能级转移,从而促进了 Pt4.1Co/KB 催化剂的 ORR 过程。这项工作为开发高效、稳健的 ORR 催化剂提供了独特的方法,从而推动了 PEMFC 的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
In situ formation of sub nanometer cobalt particle with platinum nanocrystal for high performance oxygen reduction reaction electrocatalyst
Hitherto, platinum (Pt) is the most active electrocatalyst for the oxygen reduction reaction (ORR) of the proton exchange membrane fuel cells (PEMFCs). Enhancing the performance and reducing the use of costly Pt is of great significance for the wider adoption of PEMFCs. The present research demonstrates in situ synthesized Pt nanocrystal immobilized with sub nanometer sized cobalt (Co) particles (≤ 0.3 nm) loaded on ketjenblack carbon (KB) support via a simple polyol method as a highly active ORR electrocatalyst. The as synthesized Pt4.1Co/KB catalyst featured a more positive halfwave potential of 0.925 V with a resultant of 1.8 times higher mass activity than Co free Pt/KB catalyst at 0.9 V in 0.1 M HClO4 and insignificant decay in ORR performance after 30,000 potential cycles. The excellent electrocatalytic performance of Pt4.1Co/KB has also been proven in a practical H2/air fuel cell, demonstrating a maximum peak power density of 1.08 W/cm2, comparable to the standard Pt/C-TKK (47 %) catalyst. The improved ORR performance of Pt4.1Co/KB is attributed to the incorporation of sub nanometer sized Co particles, which synergistically enhance the activity and stability. Computational studies using periodic density functional theory calculations also suggest that the integration of ultrafine Co nanoparticles shifted the Pt contribution to the density of states towards higher energy levels, thereby facilitating the ORR process for the Pt4.1Co/KB catalyst. This work provides a distinctive development of an efficient and robust ORR catalyst for advancing PEMFCs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


