掺杂 Zr 和 Y 的 SrFe0.8Zr0.1Y0.1O3-δ 包晶氧化物作为一种稳定、高性能的对称电极用于固体氧化物电池
IF 5.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
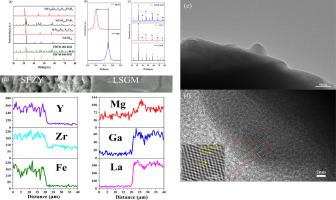
对称固体氧化物电池(SSOC)的阳极和阴极使用相同的材料,简化了电池制造工艺,并能有效防止碳沉积和硫中毒。本研究合成了 Zr、Y 共掺杂的 SrFe0.8Zr0.1Y0.1O3-δ(SFZY)包晶氧化物,并评估了其作为 SSOFCs 电极的性能。密度泛函理论(DFT)表明,SFZY 的局域态密度低于 SrFeO3-δ (SFO)。在电池工作温度以下,SFZY 与 La0.8Sr0.2Ga0.83Mg0.17O3-δ (LSGM)电解质具有良好的化学相容性。Zr、Y共掺杂的SrFe0.8Zr0.1Y0.1O3-δ在H2气氛中保持结构稳定。在 750 ℃ 时,SFZY 在空气和氢气环境中的极化电阻(Rp)分别为 0.11 和 1.06 Ω cm2。在 Rp 稳定性测试中,SFZY 在空气和氢气环境中比 SrFeO3-δ 表现出更好的稳定性。在 750 ℃ 下,以 H2 和湿 C3H8 为燃料,SFZY/LSGM/SFZY 燃料电池的最大功率密度分别达到 420.1 和 258.31 mWcm-2。在电解模式下,SFZY/LSGM/SFZY 电解池在 750 ℃ 下电解纯 CO2 时的电流密度在 1.3 V 时达到 -0.55 A cm-2。总之,SFZY 具有作为 SSOCs 电极的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Zr and Y co-doped SrFe0.8Zr0.1Y0.1O3-δ perovskite oxide as a stable, high-performance symmetrical electrode for solid oxide cells
Symmetrical solid oxide cells (SSOCs), which use the same material for both anode and cathode, simplify the cell fabrication process and effectively resist carbon deposition and sulfur poisoning. In this study, Zr, Y co-doped SrFe0.8Zr0.1Y0.1O3-δ (SFZY) perovskite oxide is synthesized and evaluated its properties as an SSOFCs electrode. Density functional theory (DFT) indicates that the local state density of SFZY is lower than that of SrFeO3-δ (SFO). SFZY has good chemical compatibility with La0.8Sr0.2Ga0.83Mg0.17O3−δ (LSGM) electrolyte below cell operating temperature. Zr, Y co-doped SrFe0.8Zr0.1Y0.1O3-δ maintains structural stability in H2 atmosphere. At 750 ℃, the polarization resistance (Rp) of SFZY is 0.11 and 1.06 Ω cm2 in air and hydrogen atmosphere, respectively. During the Rp stability test, SFZY exhibits better stability than that of SrFeO3-δ in air and hydrogen atmosphere. At 750 ℃, the maximum power density of SFZY/LSGM/SFZY fuel cell reaches 420.1 and 258.31 mWcm−2 using H2 and wet C3H8 as fuel, respectively. In electrolytic mode, the current density of SFZY/LSGM/SFZY electrolysis cell reaches -0.55 A cm−2 at 1.3 V for electrolysis of pure CO2 at 750 ℃. In summary, SFZY has a great potential as SSOCs electrode.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Research Bulletin
工程技术-材料科学:综合
CiteScore
9.80
自引率
5.60%
发文量
372
审稿时长
42 days
期刊介绍:
Materials Research Bulletin is an international journal reporting high-impact research on processing-structure-property relationships in functional materials and nanomaterials with interesting electronic, magnetic, optical, thermal, mechanical or catalytic properties. Papers purely on thermodynamics or theoretical calculations (e.g., density functional theory) do not fall within the scope of the journal unless they also demonstrate a clear link to physical properties. Topics covered include functional materials (e.g., dielectrics, pyroelectrics, piezoelectrics, ferroelectrics, relaxors, thermoelectrics, etc.); electrochemistry and solid-state ionics (e.g., photovoltaics, batteries, sensors, and fuel cells); nanomaterials, graphene, and nanocomposites; luminescence and photocatalysis; crystal-structure and defect-structure analysis; novel electronics; non-crystalline solids; flexible electronics; protein-material interactions; and polymeric ion-exchange membranes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


