H2S和近红外光驱动的纳米马达通过加强对肿瘤代谢共生的破坏,诱导二硫化氢,实现抗癌靶向治疗
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
二硫化血症是一种通过破坏肿瘤代谢共生(TMS)而导致细胞程序性死亡的新型机制,在癌症治疗中显示出巨大的潜力。然而,由于药物在实体瘤中的渗透性较差,二硫化硫的疗效受到了限制。在此,我们构建了硫化氢(H2S)和近红外(NIR)光驱动的纳米马达(称为HGPP),以有效穿透肿瘤并诱导二硫化硫。HGPP 显示了谷胱甘肽(GSH)对 H2S 的响应性释放,结合近红外光诱导的光热效应,驱动 HGPP 移动以促进肿瘤的深度穿透。释放的 H2S 会诱导肿瘤酸中毒并破坏 TMS,细胞饥饿后的二硫化物积累会导致二硫ptosis。此外,HGPP 还能诱导肝癌特异性细胞吸收,并催化葡萄糖和氧的转化,产生过氧化氢(H2O2),导致葡萄糖饥饿。总之,这项研究开发了一种多功能 Janus 纳米马达,为基于二硫化硫的实体瘤治疗提供了一种新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
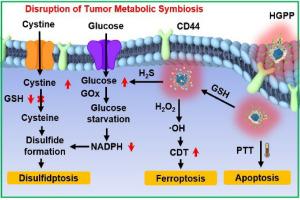
H2S and NIR light-driven nanomotors induce disulfidptosis for targeted anticancer therapy by enhancing disruption of tumor metabolic symbiosis
Disulfidptosis, a novel mechanism of programmed cell death through the disruption of tumor metabolic symbiosis (TMS), has showed tremendous potential in cancer therapy. However, the efficacy of disulfidptosis is limited by poor permeability of drugs in solid tumors. Herein, hydrogen sulfide (H2S) and near-infrared (NIR) light-driven nanomotors (denoted as HGPP) have been constructed to efficiently penetrate tumors and induce disulfidptosis. HGPP demonstrate glutathione (GSH)-responsive release of H2S, which combined with NIR light-induced photothermal effect drive HGPP movement to facilitate deep tumor penetration. The released H2S induces tumor acidosis and disrupts TMS, where disulfide accumulation following cell starvation leads to disulfidptosis. In addition, HGPP induce hepatoma specific cellular uptake and catalyze the conversion of glucose and oxygen to produce hydrogen peroxide (H2O2), leading to glucose starvation. Overall, this study has developed a multifunctional Janus nanomotor that provides a novel strategy for disulfidptosis-based solid tumor therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


