血浆代谢组学结合质谱成像揭示了胃癌发生和转移过程中肿瘤与血浆之间的相互影响
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
胃癌(GC)是一种高度致命的恶性肿瘤,预后极差。其死亡率升高的主要原因是它容易发生晚期转移。探索肿瘤微环境与全身血液之间的代谢相互作用有助于清楚地了解肿瘤生长、增殖和转移的机制并确定精确的生物标志物。本研究开发了一种将血浆代谢组学与肿瘤组织质谱成像相结合的综合方法,以研究 GC 肿瘤发生和转移的全局代谢图谱。结果显示,在非转移性 GC(M0)中,氧化谷胱甘肽与谷胱甘肽的比率(GSSH/GSH)升高,这意味着氧化应激在肿瘤组织中积累。此外,研究还发现,在远端转移性 GC(M1)的血浆和肿瘤组织中,多不饱和脂肪酸(如 9,10-EpOMe、9-HOTrE 等)的过氧化反应加快。质谱成像(MSI)和免疫组化(IHC)进一步证实了 CYP2E1 和 GGT1 对 GC 转移潜能的潜在影响。总之,我们的研究结果表明,综合多维代谢组学方法是一种临床有用的方法,可用于揭示血液与肿瘤之间的代谢串扰,阐明重新编程的代谢网络,并提供可靠的循环生物标志物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
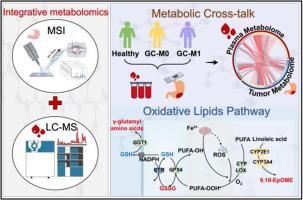
Plasma metabolomics combined with mass spectrometry imaging reveals crosstalk between tumor and plasma in gastric cancer genesis and metastasis
Gastric Carcinoma (GC) is a highly fatal malignant tumor with a poor prognosis. Its elevated mortality rates are primarily due to its proclivity for late-stage metastasis. Exploring the metabolic interactions between tumor microenvironment and the systemic bloodstream could help to clearly understand the mechanisms and identify precise biomarkers of tumor growth, proliferation, and metastasis. In this study, an integrative approach that combines plasma metabolomics with mass spectrometry imaging of tumor tissue was developed to investigate the global metabolic landscape of GC tumorigenesis and metastasis. The results showed that the oxidized glutathione to glutathione ratio (GSSH/GSH) became increased in non-distal metastatic GC (M0), which means an accumulation of oxidative stress in tumor tissues. Furthermore, it was found that the peroxidation of polyunsaturated fatty acids, such as 9,10-EpOMe, 9-HOTrE, etc., were accelerated in both plasma and tumor tissues of distal metastatic GC (M1). These changes were further confirmed the potential effect of CYP2E1 and GGT1 in metastatic potential of GC by mass spectrometry imaging (MSI) and immunohistochemistry (IHC). Collectively, our findings reveal the integrated multidimensional metabolomics approach is a clinical useful method to unravel the blood-tumor metabolic crosstalk, illuminate reprogrammed metabolic networks, and provide reliable circulating biomarkers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


