MIL-88A(Fe)/MoS2 活化过硫酸盐对 RhB 的高效降解及其机理
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
采用原位形成法制备了 MoS2/MIL-88A (Fe)复合催化剂。对材料进行了 SEM、TEM、XRD 和 XPS 表征,并用过硫酸盐(PMS)对罗丹明 B(RhB)进行了活化降解。结果表明,M-10/M1:2 对 RhB 的降解效果最好,其降解速率常数为 0.66420 min-1,降解率是其他掺杂复合催化剂的 1.89-14 倍。扫描电镜结果表明,M - 10 的硫含量最低,MoS2 花层较少,暴露的 Mo4+ 原子较多,提高了电子传递效率,加速了降解反应。M-10/M1:2 反应前后的 XPS 光谱显示,催化剂表面的 Fe3+/Fe2+ 和 Mo4+/Mo6+ 的 REDOX 反应对 PMS 的活化起了重要作用。自由基捕获实验和 ESR 测试结果表明,M-10/M1:2/PMS 反应体系中的主要活性物质为 SO4-、1O2、-O2- 和 -OH,它们只对 RhB 的降解起作用。最后,根据M-10/M1:2/PMS体系的ESR结果和反应前后XPS表征中Fe和Mo元素的变化,提出了M-10/M1:2/PMS体系的降解机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
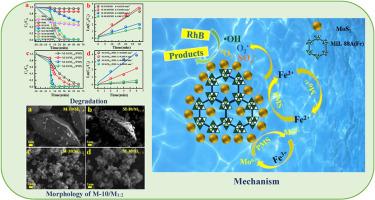
High efficiency degradation of RhB by MIL-88A(Fe)/MoS2 activated persulfate and its mechanism
MoS2/MIL-88A (Fe) composite catalyst was prepared by in-situ formation method. The material was characterized by SEM, TEM, XRD and XPS, and the Rhodamine B(RhB) was activated and degraded by persulfate (PMS). The results showed that M-10/M1:2 had the best degradation effect on RhB, the rate constant was 0.66420 min−1, and the degradation rate could reach 1.89–14 times that of other doped composite catalysts. The SEM results showed that M − 10 had the least sulfur content, fewer MoS2 flower layers and more exposed Mo4+ atoms, which improved the electron transfer efficiency and accelerated the degradation reaction. The XPS spectra before and after M-10/M1:2 reaction showed that the REDOX reaction of Fe3+/Fe2+ and Mo4+/Mo6+ on the catalyst surface played an important role in the activation of PMS. The results of free radical capture experiment and ESR test showed that the main active substances in M-10/M1:2/PMS reaction system were SO4•−, 1O2, •O2−, and •OH, which only played a role in the degradation of RhB. Finally, based on the ESR results of M-10/M1:2/PMS system and the changes of Fe and Mo elements in XPS characterization before and after the reaction, the degradation mechanism of M-10/M1:2/PMS system was proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


