含脯氨酸肽的气相质子亲和力。1:ProGly、ProAla、ProVal、ProLeu、ProIle 和 ProPro
IF 1.7
3区 化学
Q3 PHYSICS, ATOMIC, MOLECULAR & CHEMICAL
引用次数: 0
摘要
采用扩展动力学方法,在 ESI 三重四极杆仪器上测量了一系列含脯氨酸二肽的气相质子亲和力(PA)。ProGly (1)、ProAla (2)、ProVal (3)、ProLeu (4)、ProIle (5) 和 ProPro (6) 的质子亲和力分别为 969.6 ± 7.8、990.4 ± 7.7、987.6 ± 7.9、982.8 ± 8.0、988.8 ± 10.1 和 996.5 ± 12.2 kJ/mol。通过在 B3LYP/6-311++G(d,p)//B3LYP/6-31+G(d) 理论水平上进行等效计算,还得到了 1-6 质子亲和力的预测值。预测 1 和 6 的质子亲和力分别为 966.9 和 991.0 kJ/mol,与实验值一致。然而,2-5 的质子亲和力预测值为 973.5、975.9、975.7 和 975.9,比实验值低 8 至 15 kJ/mol。使用更大的基集(B3LYP/6-311++G(2df,2p))、包含色散(B3LYP-D3/6-311++G(d,p))、切换到二阶扰动理论(MP2/6-31++G(d、p)和 MP2/6-311++G(2df,2p) 或切换密度泛函(M06-2x/6-311++G(d,p) 和 M06-2x/6-311++G(2df,2p)),结果表明推导出的热化学结果变化不大,支持了原始计算。我们建议使用 ProGly 和 ProPro 的实验质子亲和力,并使用 ProAla、ProVal、ProLeu 和 ProIle 的计算值以及实验质子亲和力作为上限。本文章由计算机程序翻译,如有差异,请以英文原文为准。
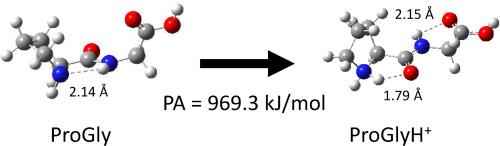
Gas phase proton affinities of proline-containing peptides. 1: ProGly, ProAla, ProVal, ProLeu, ProIle, and ProPro
The gas-phase proton affinities (PA) for a series of proline-containing dipeptides have been measured in an ESI triple quadrupole instrument using the extended kinetic method. Proton affinities for ProGly (1), ProAla (2), ProVal (3), ProLeu (4), ProIle (5), and ProPro (6) were determined to be 969.6 ± 7.8, 990.4 ± 7.7, 987.6 ± 7.9, 982.8 ± 8.0, 988.8 ± 10.1, and 996.5 ± 12.2 kJ/mol, respectively. Predictions for the proton affinities for 1–6 were also obtained through isodesmic calculations at the B3LYP/6-311++G(d,p)//B3LYP/6-31+G(d) level of theory. The predicted proton affinities for 1 and 6 of 966.9 and 991.0 kJ/mol are in agreement with the experimental values. However, the predicted proton affinities for 2–5 of 973.5, 975.9, 975.7, and 975.9 are between 8 and 15 kJ/mol lower than the experimental values. Additional calculations with a larger basis set (B3LYP/6-311++G(2df,2p), inclusion of dispersion (B3LYP-D3/6-311++G(d,p)), switching to second order perturbation theory (MP2/6-31++G(d,p) and MP2/6-311++G(2df,2p), or switching density functional (M06-2x/6-311++G(d,p) and M06-2x/6-311++G(2df,2p) show only modest changes in derived thermochemistry lending support to the original calculations. We recommend using the experimental proton affinities for ProGly and ProPro and using the calculated values for ProAla, ProVal, ProLeu, and ProIle with the experimental proton affinities as upper limits.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.60
自引率
5.60%
发文量
145
审稿时长
71 days
期刊介绍:
The journal invites papers that advance the field of mass spectrometry by exploring fundamental aspects of ion processes using both the experimental and theoretical approaches, developing new instrumentation and experimental strategies for chemical analysis using mass spectrometry, developing new computational strategies for data interpretation and integration, reporting new applications of mass spectrometry and hyphenated techniques in biology, chemistry, geology, and physics.
Papers, in which standard mass spectrometry techniques are used for analysis will not be considered.
IJMS publishes full-length articles, short communications, reviews, and feature articles including young scientist features.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


