生物制药干燥技术的过程控制和设计 - 综述
IF 4.5
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
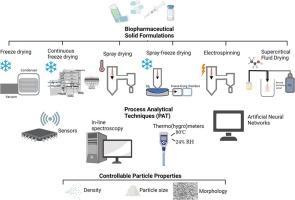
近十年来,固态生物大分子的生产研究与日俱增,发现了新的给药途径并提高了产品的稳定性。冷冻干燥是生物分子脱水最常用的工业方法,但这种方法需要较长的加工时间,而且无法进行颗粒工程。因此,人们不断开发新的干燥技术,以生产干燥的生物制药,促进从批量生产到连续生产的转变,并改善对颗粒属性的控制。生物产品的敏感性要求对这些新方法进行全面优化,以应对干燥带来的各种降解压力。工艺控制和优化是最大限度地减少其中许多应力的关键,这样才能生产出具有预定特性(如颗粒形态、大小和密度)的干燥粉末。在本综述中,我们将详细介绍迄今为止用于生物制剂干燥的现有方法、这些方法的颗粒工程能力以及工艺分析技术 (PAT) 带来的工艺控制可能性。我们还探讨了质量和能量平衡对工艺优化的影响,以及工艺控制对生物大分子稳定性、干燥效率和颗粒工程学的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Process control and design of drying technologies for biopharmaceuticals – A review
Research into the production of solid-state biomolecules has increased in the last decade, uncovering new routes of administration and enhanced product stability. Freeze drying is the most common industrial method for biomolecule dehydration, however it requires long processing times and does not allow for particle engineering. Hence, new drying techniques are constantly being developed to produce dried biopharmaceuticals, facilitating the switch from batch to continuous manufacturing and improving control over particle attributes. The sensitive nature of biological products requires comprehensive optimisation of these new methods against the various degradative stresses imposed by drying. Process control and optimisation is key in minimizing many of these stresses, allowing production of dried powders with pre-determined characteristics (e.g. particle morphology, size and density). In this review, we provide a detailed overview of current methods used to date for the drying of biologics and the particle engineering capabilities of these methods, along with the process control possibilities that emerge with process analytical technology (PAT). We also look at the extent of mass and energy balances informing process optimisation and the effect of process controls on biomolecule stability, drying efficiency, and particle engineering.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Powder Technology
工程技术-工程:化工
CiteScore
9.90
自引率
15.40%
发文量
1047
审稿时长
46 days
期刊介绍:
Powder Technology is an International Journal on the Science and Technology of Wet and Dry Particulate Systems. Powder Technology publishes papers on all aspects of the formation of particles and their characterisation and on the study of systems containing particulate solids. No limitation is imposed on the size of the particles, which may range from nanometre scale, as in pigments or aerosols, to that of mined or quarried materials. The following list of topics is not intended to be comprehensive, but rather to indicate typical subjects which fall within the scope of the journal's interests:
Formation and synthesis of particles by precipitation and other methods.
Modification of particles by agglomeration, coating, comminution and attrition.
Characterisation of the size, shape, surface area, pore structure and strength of particles and agglomerates (including the origins and effects of inter particle forces).
Packing, failure, flow and permeability of assemblies of particles.
Particle-particle interactions and suspension rheology.
Handling and processing operations such as slurry flow, fluidization, pneumatic conveying.
Interactions between particles and their environment, including delivery of particulate products to the body.
Applications of particle technology in production of pharmaceuticals, chemicals, foods, pigments, structural, and functional materials and in environmental and energy related matters.
For materials-oriented contributions we are looking for articles revealing the effect of particle/powder characteristics (size, morphology and composition, in that order) on material performance or functionality and, ideally, comparison to any industrial standard.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


