聚碳酸酯与石墨烯和氧化硅的界面粘附力:分子动力学对比分析
IF 5.7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
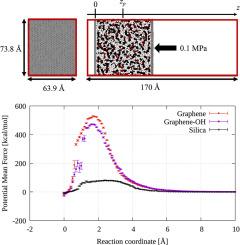
本研究采用分子动力学方法来评估聚碳酸酯在代表纤维材料的固体壁面上的无吸附能。使用了三种固体表面:不含官能团的清洁石墨烯(代表表面处理过的碳材料)、不含表面处理过的带羟基的石墨烯以及代表玻璃纤维的二氧化硅。结果表明,清洁石墨烯的单位面积吸附自由能大于其他两种固体壁面的单位面积吸附自由能,这与实验结果不同,表面处理过的碳纤维的吸附自由能较小。对苯环的结构进行分析后发现,苯环在所有实体壁上几乎都是平行于实体壁沉积的,而在石墨烯上,带有 SiO2 和 OH 基团的苯环密度最小,角度最小,最靠近实体壁,因此吸附能较小。这表明,苯环与固体表面之间的 π-π 相互作用可能占吸附能的很大一部分。这还表明,固体表面与聚合物之间的化学反应可能对吸附能有重大影响,而本研究并未考虑这些反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Interfacial adhesion of polycarbonate to graphene and silicon oxide: A comparative molecular dynamics analysis
In this study, molecular dynamics was used to evaluate the adsorption-free energy of polycarbonate on a solid wall surface that represented a fiber material. Three solid surfaces were used: clean graphene without functional groups to represent surface-treated carbon materials, graphene with OH groups without surface treatment, and SiO2 to represent glass fiber. The results showed that the adsorption free energy per unit area for clean graphene was greater than the adsorption free energy per unit area for the other two solid wall surfaces, which is different from the experimental results where the surface treated carbon fiber had a smaller adsorption free energy. The structure of benzene rings was analyzed, and it was found that benzene rings are deposited almost parallel to the solid wall on all solid walls and that the density of benzene rings with SiO2 and OH groups is the smallest on Graphene with the smallest angle and closest to the solid wall, resulting in smaller adsorption energy. This suggests that the π-π interaction between the benzene ring and the solid surface may account for a large proportion of the adsorption energy. It also suggests that chemical reactions between the solid surface and the polymer, which were not considered in this study, may have a significant effect on the adsorption energy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


