噻吩系列 CF3-配位物作为合成新型 CF3-噻吩的关键中间体的性质的 DFT 计算
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
噻吩-2-基-(三氟甲基)甲醇的三甲基硅醚在布氏超酸性酸 CF3SO3H 的作用下发生多通道亲电转化,形成多种 CF3 取代的噻吩结构。这些转化的关键阳离子中间体是 CF3-配位体。利用密度泛函理论(DFT)计算了这些关键中间产物的电子和轨道特征、全局亲电指数以及热力学特征。量子化学计算的结果解释了中间阳离子物种反应性的实验数据。结果表明,阳离子与芳香族亲核物的反应主要受轨道控制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
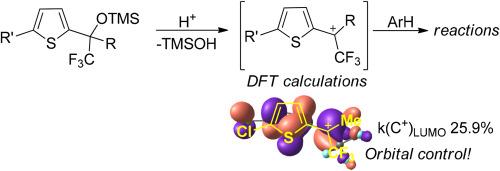
DFT calculations of properties of CF3-carbocations of the thiophene series as key intermediates in the synthesis of novel CF3-thiophenes
Trimethylsilyl ethers of thiophen-2-yl-(trifluoromethyl)methanols undergo multichannel electrophilic transformations under the action of Brønsted superacid acid CF3SO3H to form a wide range of CF3-substituted thiophene structures. The key cationic intermediates of these transformations are CF3-carbocations. Electronic and orbital characteristics, global electrophilicity indices, and thermodynamic characteristics of these key intermediates have been calculated with a use of the density functional theory (DFT). Experimental data on the reactivity of intermediate cationic species have been explained on the basis of the results of quantum chemical calculations. It has been shown that reactions of the cations with aromatic nucleophiles are mainly ruled by orbital control.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


