二维共培养模型揭示了活化成纤维细胞与癌细胞之间的生物物理相互作用。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
肿瘤微环境(TME)由改变了的细胞外基质(ECM)中的各种细胞类型组成,通过细胞-细胞和细胞-ECM之间错综复杂的相互作用在转移中发挥着关键作用。成纤维细胞作为细胞外基质的关键成分,通过参与基质沉积和重塑机制,在静止或活化状态的调节下,对癌症转移做出了重要贡献。尽管成纤维细胞的重要性已得到公认,但人们对其在癌细胞侵袭中的确切作用仍不甚了解。在本研究中,我们使用二维共培养模型研究了成纤维细胞活性对癌细胞进展的影响。将密歇根癌症基金会-7(MCF7)乳腺癌细胞与正常人肺成纤维细胞(NHLF)共培养,无论是否经过转化生长因子β(TGFβ)处理。我们采用了牵引力显微镜(TFM)来量化与细胞迁移相关的牵引力和速度力。我们观察到,在共培养过程中,TGFβ激活的成纤维细胞在癌细胞周围形成一个独特的环,细胞岛边界的牵引力和张力增加。这种力的分布与力相关蛋白在这些边界区域的定位有关,包括 vinculin 和 E-cadherin。代谢谱分析显示,活化的成纤维细胞特有强烈的 OXPHOS 信号,这与正常成纤维细胞形成鲜明对比,正常成纤维细胞主要表现出迁移行为,而在共培养过程中,成纤维细胞的受力和代谢活动模式更不均匀。我们的发现为研究肿瘤微环境中支配细胞迁移的机械力和代谢动力学提供了宝贵的见解,我们的共培养模型可以补充体内研究,使研究人员能够探索特定的微环境线索,从而更深入地了解肿瘤微环境的机制。意义说明:模拟肿瘤微环境(TME)动态的癌症模型是研究癌症机制和治疗的理想工具。然而,人们对癌细胞如何与其周围环境和其他细胞相互作用仍一无所知。为了解决这个问题,我们开发了一种简单而有效的二维共培养模型,它允许我们精确控制细胞培养物的排列,并使用各种成像技术来研究癌细胞与成纤维细胞之间的相互作用。在这里,我们可以在体外测量细胞运动、力分布、代谢活动和蛋白质定位以及这些因素之间的相互作用。我们的模型有助于我们观察癌细胞和成纤维细胞之间的内在机制,从而加深我们对肿瘤组织间质动态的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
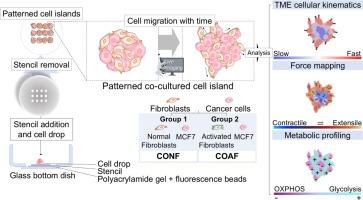
2D co-culture model reveals a biophysical interplay between activated fibroblasts and cancer cells
The tumor microenvironment (TME) comprises diverse cell types within an altered extracellular matrix (ECM) and plays a pivotal role in metastasis through intricate cell-cell and cell-ECM interactions. Fibroblasts, as key constituents of the TME, contribute significantly to cancer metastasis through their involvement in matrix deposition and remodeling mechanisms, modulated by their quiescent or activated states. Despite their recognized importance, the precise role of fibroblasts in cancer cell invasion remains incompletely understood. In this study, we investigated the impact of fibroblast activity on cancer cell progression using a 2D co-culture model. Michigan Cancer Foundation-7 (MCF7) breast cancer cells were co-cultured with normal human lung fibroblasts (NHLF), both with and without transforming growth factor β (TGFβ) treatment. Traction force microscopy (TFM) was employed to quantify traction and velocity forces associated with cellular migration. We observed that TGFβ-activated fibroblasts form a distinctive ring around cancer cells in co-culture, with increased traction and tension at the cell island boundary. This force distribution is associated with the localization of force-related proteins at these boundary regions, including vinculin and E-cadherin. Metabolic profiling revealed a strong OXPHOS signal specific to the activated fibroblasts, in contrast to normal fibroblasts, which primarily display migratory behavior and a more heterogeneous pattern of forces and metabolic activity in co-culture. Our findings offer valuable insights into the mechanical forces and metabolic dynamics governing cellular migration in the tumor microenvironment, where our co-culture model could complement in vivo studies and enable researchers to explore specific microenvironmental cues for a deeper understanding of TME mechanisms.
Statement of significance
Cancer models mimicking the dynamics of tumor microenvironment (TME) are an ideal tool to study cancer mechanisms and treatment. However, the full understanding of how cancer cells interact with their surroundings and other cells is still unknown. To tackle this, we developed a simple yet effective 2D co-culture model that allows us to control the arrangement of cell cultures precisely and use various imaging techniques to study interactions between cancer cells and fibroblasts. Here we could measure cell movements, force distribution, metabolic activity, and protein localization and interplay those factors in vitro. Our model helps us observe the underlying mechanisms between cancer cells and fibroblasts, contributing to our understanding of the dynamics in the TME.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


