氟喹诺酮类抗生素环丙沙星在水溶液中的紫外线光降解机理。
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
通过结合纳秒激光闪烁光解、稳态光解以及高分辨率液相色谱-质谱和 DFT 量子化学计算,揭示了喹诺酮类抗生素环丙沙星 (CIP) 齐聚物和阴离子形式的直接紫外光解机理。这两种形式的主要中间体都是离解三重态,失去一个氟离子后形成三重碳位;后者随后受到溶剂侵蚀,形成芳香环和哌嗪取代基的羟基化产物。相应地,两种 CIP 形式的光解量子产率并不取决于激发波长,而是取决于溶解氧的浓度。二次光解会产生多种芳香系统氧化产物,以及哌嗪基取代基的氧化、开放和完全破坏。所获得的结果可能对了解喹诺酮类抗生素在紫外线消毒过程中以及在阳光作用下在自然水体中的命运具有重要意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
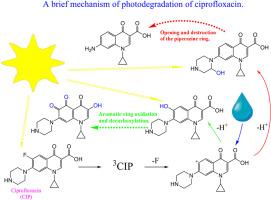
Mechanism of UV photodegradation of fluoroquinolone antibiotic ciprofloxacin in aqueous solutions
Mechanism of direct UV photolysis of the zwitterionic and anionic forms of the quinolone antibiotic ciprofloxacin (CIP) was revealed by combination of nanosecond laser flash photolysis, steady-state photolysis coupled with high resolution LC-MS and DFT quantum-chemical calculations. For both forms, the main intermediate is a dissociative triplet state, which loses a fluorine ion to form a triplet carbocation; subsequent solvent attack of the latter leads to the formation of products of hydroxylation both the aromatic ring and the piperazyl substituent. Correspondingly, the quantum yield of photolysis of both CIP forms does not depend on the excitation wavelength, but depends on the concentration of dissolved oxygen. Secondary photolysis leads to a number of products of oxidation of the aromatic system, as well as oxidation, opening and full destruction of the piperazinyl substituent. The results obtained may be important for understanding the fate of quinolone antibiotics in UVC disinfection processes and in natural waters under the action of sunlight.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


