硬脂酸基纳米颗粒负载抗菌肽 - Bacitracin 和 LL-37:制造参数、细胞相容性和抗菌功效的选择。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
固体脂质纳米颗粒是目前研究最为广泛的药物输送载体类型之一。考虑到两种 COVID-19 mRNA 疫苗获批后促进了它们的临床转化,充分解释加工参数如何影响所获纳米颗粒的特性和药物负载效率至关重要。本研究旨在评估不同生产参数对使用乳化/溶剂扩散法制造的硬脂酸基纳米粒子性能的影响。研究发现,使用的有机溶剂类型对纳米粒子的形态有很大影响,氯仿适合生产球形纳米粒子。纳米颗粒的大小和多分散性受外部水相中表面活性剂的浓度、有机相中硬脂酸的浓度以及均质振幅的影响。优化后的纳米颗粒成功载入了抗菌肽 LL-37。LL-37 的存在对纳米颗粒的形态和细胞相容性没有明显影响。所获得的纳米颗粒对化脓性链球菌(ATCC 12384)参考菌株具有抗菌活性。所开发的固体脂质纳米粒子是一种很有前景的药物载体,可进一步优化用于治疗受感染的伤口或呼吸系统的细菌感染。本文章由计算机程序翻译,如有差异,请以英文原文为准。
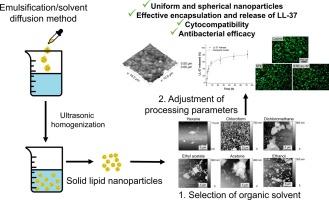
Stearic acid-based nanoparticles loaded with antibacterial peptides – Bacitracin and LL-37: Selection of manufacturing parameters, cytocompatibility, and antibacterial efficacy
Solid lipid nanoparticles are currently one of the most widely investigated types of drug delivery carriers. Considering the fact that their clinical translation boosted after the approval of two COVID-19 mRNA vaccines, it is crucial to fully explain how the processing parameters affect the properties of the obtained nanoparticles and the drug loading efficiency. This study aimed to evaluate the influence of different manufacturing parameters on the properties of stearic acid-based nanoparticles fabricated using the emulsification/solvent diffusion method. It was found that the type of organic solvent used has a major effect on the morphology of the nanoparticles, with chloroform being suitable for the production of spherical nanoparticles. The size and polydispersity of the nanoparticles were affected by the concentration of surfactant in the external aqueous phase, the concentration of stearic acid in the organic phase, and the homogenization amplitude. The optimized nanoparticles were successfully loaded with an antibacterial peptide – LL-37. The presence of LL-37 did not significantly influence nanoparticle morphology or cytocompatibility. The obtained nanoparticles showed antibacterial activity against the reference strain of Streptococcus pyogenes (ATCC 12384). The developed solid lipid nanoparticles are promising drug carries that can be further optimized for the treatment of infected wounds or bacterial infections in the respiratory system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


