模拟藻酸低聚物在外力作用下的构象变化。
IF 2.4
3区 化学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
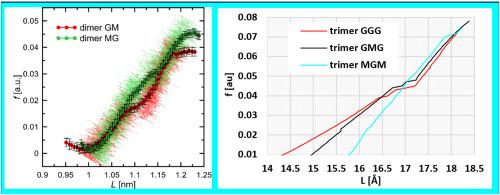
本研究探讨了不同比例的β-d-甘露糖酸(M单位)和α-l-谷朊酸(G单位)单位的海藻酸低聚物的机械力诱导构象转变的机制和性质。研究还考虑了同源或异源低聚物中糖苷键的类型对构象转变性质的影响。为此,研究人员采用了两种不同的理论方法:DFT 水平的量子力学(QM)和 EGO(强制几何优化)方法,前者曾在其他糖类系统中进行过测试;后者是在混合相互作用势中进行的分子动力学(MD)模拟,其中考虑到了 ab initio(QM)理论水平和经典分子力学(MM)力场。这样就能详细描述在外部机械力影响下发生的构象转变的结构和能量特性(例如,环形反转过程路径上的环形构象,以及与初始、最终和中间状态相对应的能量)。研究结果表明,G:M 比率不同,对外力的反应也不同,其根源在于 G 或 M 单元中糖苷键的拓扑结构不同。这对于通过原子力显微镜实验研究确定天然异构藻酸盐链的含量具有潜在意义。显性溶剂和非零温度的影响对于确定拉伸特性并不重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Modeling conformational changes in alginic acid oligomers induced by external forces
In this study, the mechanism and nature of mechanical force-induced conformational transitions of alginate oligomers with different ratios of β-d-mannuronic acid (M unit) and α-l-guluronic acid (G unit) units were investigated. The influence of the type of glycosidic linkage in either homo- or heterooligomers on the nature of conformational transitions was also considered. For this purpose, two different theoretical methods were used: quantum mechanics (QM) at the DFT level with the EGO (Enforced Geometry Optimization) approach previously tested also for other saccharide systems, and molecular dynamics (MD) simulations within hybrid interaction potentials, which take into account both the ab initio (QM) level of theory and classical molecular mechanics (MM) force fields. This allowed to characterize in detail the structural and energetic properties of the conformational transition occurring upon the influence of external, mechanical forces (e.g. ring conformations at the path of ring-inversion process as well as the energies corresponding to initial, final and intermediate states). The results indicate qualitatively various responses against the applied force, depending on the G:M ratio, which have their source in differing topologies of glycosidic linkage in either G or M units. This is of potential relevance for determining the content of naturally heterogeneous alginate chains by the AFM experimental studies. The effects of explicit solvent and non-zero temperature are not of primarily importance in the context of determined stretching properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Research
化学-生化与分子生物学
CiteScore
5.00
自引率
3.20%
发文量
183
审稿时长
3.6 weeks
期刊介绍:
Carbohydrate Research publishes reports of original research in the following areas of carbohydrate science: action of enzymes, analytical chemistry, biochemistry (biosynthesis, degradation, structural and functional biochemistry, conformation, molecular recognition, enzyme mechanisms, carbohydrate-processing enzymes, including glycosidases and glycosyltransferases), chemical synthesis, isolation of natural products, physicochemical studies, reactions and their mechanisms, the study of structures and stereochemistry, and technological aspects.
Papers on polysaccharides should have a "molecular" component; that is a paper on new or modified polysaccharides should include structural information and characterization in addition to the usual studies of rheological properties and the like. A paper on a new, naturally occurring polysaccharide should include structural information, defining monosaccharide components and linkage sequence.
Papers devoted wholly or partly to X-ray crystallographic studies, or to computational aspects (molecular mechanics or molecular orbital calculations, simulations via molecular dynamics), will be considered if they meet certain criteria. For computational papers the requirements are that the methods used be specified in sufficient detail to permit replication of the results, and that the conclusions be shown to have relevance to experimental observations - the authors'' own data or data from the literature. Specific directions for the presentation of X-ray data are given below under Results and "discussion".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


