硼烷硫醇钯催化合成硼烷硫化物。
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
硼烷是一类有趣的芳香族分子,具有二十面体几何形状、高稳定性和独特的电子效应。我们在此报告了钯催化的硼烷硫醇与芳基卤化物的偶联反应。该方案适用于受控合成二(碳硼酰)硫化物,并考察了它们在芳香卤化反应中的催化性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
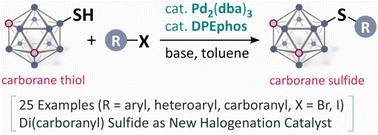
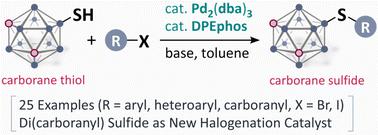
Pd-Catalysed synthesis of carborane sulfides from carborane thiols†
Carboranes are an interesting class of aromatic molecules with icosahedral geometry, high stability, and unique electronic effects. We herein report a Pd-catalysed coupling reaction of carborane thiols with aryl halides. This protocol was applicable to the controlled synthesis of di(carboranyl) sulfides, and their catalytic performance for aromatic halogenation was examined.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


