紫外线/氯处理过程中Cl/N峰值恒定的含氮消毒副产物的Wane-and-wax机理:对新饮用水消毒策略的启示。
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
含氮消毒副产物(N-DBPs)因其严重的健康风险而臭名昭著,但硝酸盐(NO3-)在紫外线/氯处理过程中介导 N-DBPs 生成的机制仍未得到研究。本研究调查了氯和 NO3- 对 N-DBPs 生成的相互作用,并利用 UPLC-QTOF-MS 开发了一种基于特定片段的筛选方法来探索其潜在机制。结果表明,氯氮(Cl/NO3--N)摩尔比会显著影响二氯乙腈(DCAN)和二氯乙酰胺(DCAM)的生成,在 Cl/NO3-N 摩尔比为 15 左右时会达到峰值浓度。NO3- 会促进 HO- 的生成,而 HO- 的生成与 DCAN 和 DCAM 的浓度呈正相关,同样在此比率下达到峰值。利用我们开发的方法,确定了三个关键的羟基取代中间体,它们绕过了之前报道的 DCAN 形成过程中的 "限制步骤"。该反应通过羟基化和氯取代的逐步机制进行,生成羟基-苯乙腈和羟基-氯-苯乙腈。在 Cl/NO3-N 摩尔比为 15 的条件下,羟基-氯-苯乙腈转化为 DCAN 的速率是不含 NO3-N 条件下的 8.6 倍,这归因于侧链的键强度减弱,密度泛函理论计算也证明了这一点。这项研究提供了有关 DCAN 和 DCAM 形成机理途径的新见解,对于开发更有效的饮用水消毒技术以控制 N-DBPs 至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
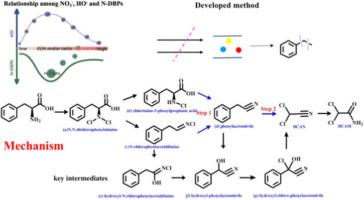
Wane-and-wax mechanism of nitrogenous disinfection byproducts with constant Cl/N peak under UV/chlorine treatment: Implication for new drinking water disinfection strategy
Nitrogenous disinfection byproducts (N-DBPs) are notorious for their serious health risks, yet nitrate (NO3-) mediates N-DBPs generation during UV/chlorine treatment remains unexplored. This study investigated the interaction of chlorine and NO3- on N-DBPs formation and developed a specific fragment-based screening method using UPLC-QTOF-MS to explore the underlying mechanism. Results showed that the chlorine-to-nitrogen (Cl/NO3--N) molar ratio significantly affects dichloroacetonitrile (DCAN) and dichloroacetamide (DCAM) generation, with peak concentrations at a Cl/NO3--N molar ratio of around 15. NO3- promotes the production of HO•, which positively correlates with DCAN and DCAM concentrations, also peaking at this ratio. Utilizing our developed method, three key hydroxyl-substituted intermediates that circumvent the previously reported “limiting-steps” in DCAN formation were identified. This reaction proceeds via a stepwise mechanism involving hydroxylation and chlorine substitution to produce hydroxyl-phenylacetonitrile and hydroxyl-chlorine-phenylacetonitrile. The conversion rate of hydroxyl-chlorine-phenylacetonitrile to DCAN was 8.6 times higher at Cl/NO3--N molar ratio of 15 compared to conditions without NO3-, attributed to the weakened bond strength of the side chain, as supported by density functional theory calculations. This study provides novel insights into the mechanistic pathways of DCAN and DCAM formation, critical for developing more effective drinking water disinfection technologies to control N-DBPs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


