用于丙烷氧化脱氢的单原子锰基催化剂
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
通过高通量合成平台和平行反应器,对 150 种支撑金属氧化物(锰和添加剂)催化剂进行了组合筛选,用于丙烷氧化脱氢(ODH)制丙烯。具体来说,有机锰(0.05-2.5 个锰原子/nm2)复合物被接枝到金属氧化物载体(Al2O3、SiO2、TiO2 和 ZrO2)上,并预先用路易斯酸(Al、Ti、Zn 和 Zr)或氧化还原活性(Cu、Cr、Ga Ni、V)添加剂修饰,表面覆盖率各不相同(25%、50% 和 75%)。催化剂的表征方法包括高分辨率透射电子显微镜 (HRTEM)、X 射线光电子能谱 (XPS)、X 射线衍射 (XRD)、拉曼光谱和紫外可见光谱。0.05 Mn/V(50%)/Al2O3 和 0.05 Mn/Ni(50%)/ZrO2 催化剂的丙烷综合转化率和丙烯选择性最高(31/41% 和 15/85%),在 500 °C 下 25 小时内稳定性极佳。在 500 °C 热处理后,HRTEM 发现了单个锰原子。在 Mn/Ni/ZrO2 系统中,由于 Mn2+ 和 Zr4+ 的离子半径相似,锰被纳入了支撑晶格,而镍的存在也增强了锰的结合。在 Mn/V/Al2O3 体系中,拉曼观测到高活性氧化锰非常普遍。V 和 Mn 都促进了相互分散的增加,但这两种物质都留在了表面。据推测,高度分散的原子以及锰与镍或钒之间的相互作用是 ODH 性能和稳定性的原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
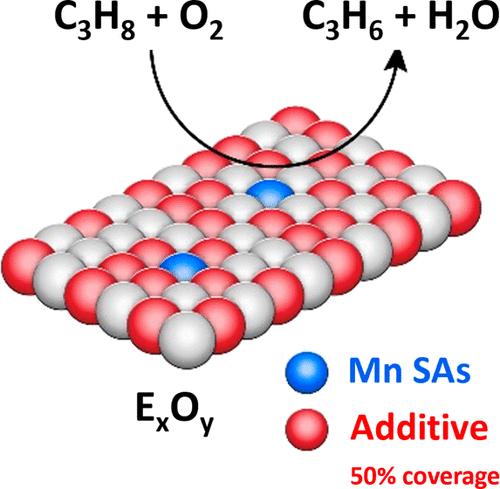
Single-Atom Manganese-Based Catalysts for the Oxidative Dehydrogenation of Propane
Combinatorial screening of 150 supported metal oxide (manganese and additives) catalysts was carried out via a high-throughput synthesis platform and parallel reactors for the oxidative dehydrogenation (ODH) of propane to propylene. Specifically, an organomanganese (0.05–2.5 Mn atoms/nm2) complex was grafted on metal oxide supports (Al2O3, SiO2, TiO2, and ZrO2) premodified with either Lewis acid (Al, Ti, Zn, and Zr) or redox-active (Cu, Cr, Ga Ni, V) additives at various surface coverages (25, 50, and 75%). Catalysts were characterized by high-resolution transmission electron microscopy (HRTEM), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), Raman spectroscopy, and UV–vis spectroscopy. Catalysts 0.05 Mn/V(50%)/Al2O3 and 0.05 Mn/Ni(50%)/ZrO2 showed the highest combined propane conversion and propylene selectivities (31/41% and 15/85%), with excellent stability at 500 °C for 25 h. The presence of Ni in Mn/Ni/ZrO2 resulted in a 6-fold increase in turnover frequency (TOF) over the Mn/ZrO2. HRTEM identified single Mn atoms after 500 °C heat treatment. For the Mn/Ni/ZrO2 system, Mn was incorporated into the support lattice due to the similar ionic radius of Mn2+ and Zr4+, which was also enhanced by the presence of Ni. For the Mn/V/Al2O3 system, highly active MnO was prevalent as observed by Raman. Both V and Mn contributed to an increase in mutual dispersion, but both species remained on the surface. It is proposed that the highly dispersed atom and interactions between Mn with either Ni or V are responsible for the ODH performance and stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


