利用绿色深共晶溶剂从废旧锂离子电池中高效萃取有价金属:工艺优化、机理分析和环境影响评估
IF 9.7
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
到 2030 年,废旧锂离子电池(LIB)的累积量将超过 1100 万吨,这凸显了大规模淘汰锂离子电池所带来的严峻环境挑战。从废旧锂离子电池中有效回收有价值的金属,既能减少对环境的影响,又能缓解金属资源稀缺的紧迫问题。在此背景下,深共晶溶剂(DES)作为一种生态友好型溶剂已成为一种前景广阔的选择,在回收废锂电池方面展现出巨大的潜力。因此,本研究提出了一种由氯化胆碱(ChCl)、DL-苹果酸(MAL)和甘油组成的创新型绿色 DES 系统,用于从废锂电池中高效沥滤有价金属。通过综合实验确定了最佳浸出条件(130 °C, 60 g/L, MChCl:MAL:Glycerol of 1:1:3, 3 h),镍的浸出效率高达 94.6%,钴的浸出效率高达 96.8%,锰的浸出效率高达 93.8%,锂的浸出效率高达 96.4%。通过表征技术、动力学研究和密度泛函理论(DFT)计算,镍、钴、锰和锂的浸出过程主要受收缩核心模型中的表面化学反应(1-(1-x)1/3=kt)控制,活化能分别为 49.89 kJ/mol、47.66 kJ/mol、50.51 kJ/mol 和 22.24 kJ/mol。DES 浸出金属离子的倾向性依次为根据结合能和能隙确定,DES 对金属离子的浸出倾向依次为:Li > Co > Ni > Mn。DES 中的 Cl- 和 -COOH 能够与还原过渡金属离子形成稳定的络合物,揭示了一种高效的配位浸出机制。此外,还对 DES 沥滤过程的环境影响进行了生命周期评估 (LCA),证实它是回收废 LIB 的一种有效且环保的方法。这项工作避免了腐蚀性酸的使用,缓解了 DESs 沥滤过程中普遍存在的恶劣条件,为回收废 LIB 提供了一个可行的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
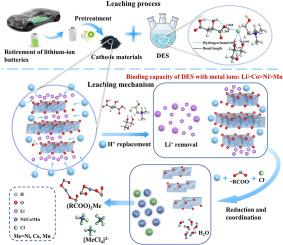
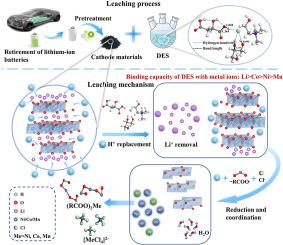
Efficient leaching of valuable metals from spent lithium-ion batteries using green deep eutectic solvents: Process optimization, mechanistic analysis, and environmental impact assessment
The accumulation of over 11 million tons of spent lithium-ion batteries (LIBs) by 2030 highlights a critical environmental challenge posed by their large-scale retirement. The efficient recycling valuable metals from spent LIBs can both reduces environmental impact and mitigates the pressing issue of metal resource scarcity. In this context, deep eutectic solvents (DESs) have become a promising option as an eco-friendly solvent, exhibiting great potential for recycling spent LIBs. Therefore, this work proposed an innovative green DES system consisting of choline chloride (ChCl), DL-malic acid (MAL), and glycerol for the efficient leaching of valuable metals from spent LIBs. Comprehensive experiments were conducted to determine the optimum leaching conditions (130 °C, 60 g/L, MChCl:MAL:Glycerol of 1:1:3, 3 h), achieving high leaching efficiencies of 94.6% for Ni, 96.8% for Co, 93.8% for Mn, and 96.4% for Li. Through characterization techniques, kinetics studies, and density functional theory (DFT) calculations, the leaching process was predominantly governed by surface chemical reaction (1-(1-x)1/3 = kt) within the shrinking core model, exhibited activation energies of 49.89 kJ/mol, 47.66 kJ/mol, 50.51 kJ/mol, and 22.24 kJ/mol for Ni, Co, Mn, and Li, respectively. The propensity for DES to leach metal ions followed the order: Li > Co > Ni > Mn, determined by binding energy and energy gaps. Cl− and −COOH within the DES were capable of forming stable complexes with reduced transition metal ions, revealing an efficient coordination leaching mechanism. Additionally, a life cycle assessment (LCA) was conducted on the environmental impacts of the DES leaching process, confirming it as an effective and environmentally friendly method for recycling spent LIBs. This work avoided the employment of corrosive acids and alleviated the generally harsh conditions associated with DESs leaching, providing a viable solution for recovering spent LIBs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Cleaner Production
环境科学-工程:环境
CiteScore
20.40
自引率
9.00%
发文量
4720
审稿时长
111 days
期刊介绍:
The Journal of Cleaner Production is an international, transdisciplinary journal that addresses and discusses theoretical and practical Cleaner Production, Environmental, and Sustainability issues. It aims to help societies become more sustainable by focusing on the concept of 'Cleaner Production', which aims at preventing waste production and increasing efficiencies in energy, water, resources, and human capital use. The journal serves as a platform for corporations, governments, education institutions, regions, and societies to engage in discussions and research related to Cleaner Production, environmental, and sustainability practices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


