光化学氢化脱硫中的三(三甲基硅基)硅烷--方法和发火性
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
我们开发了一种新型可见光诱导的硫代缩醛加氢脱硫反应。该反应使用方便的三(三甲基硅基)硅烷作为还原剂,光活性 4CzIPN 的催化剂载量较低,反应条件温和。反应范围的扩大受阻于该工艺的操作危险性,偶尔会产生火灾。对反应混合物进行仔细检查后,确定硅烷(SiH4)可能是导致发火的罪魁祸首。本文章由计算机程序翻译,如有差异,请以英文原文为准。
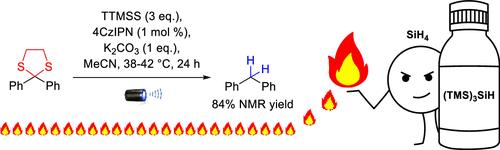
Tris(trimethylsilyl)silane in Photochemical Hydrodesulfurization─Methodology and Pyrophoricity
A novel visible-light-induced hydrodesulfurization of a thioacetal was developed. The reaction operates under mild conditions using user-friendly tris(trimethylsilyl)silane as the reductant and a low catalyst loading of photoactive 4CzIPN. The expansion of the reaction scope was thwarted by the operationally hazardous nature of the process, occasionally producing fire. Careful examination of reaction mixtures allowed to identify silane (SiH4) as the likely culprit causing the pyrophoricity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


