基于烯基吲哚-DCAF11 对的 BRD4 PROTAC 的设计、合成和活性评估
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
蛋白水解靶向嵌合体(PROTAC)是靶向不可药用蛋白的一种先进策略,而靶向 E3 连接酶的分子弹头起着至关重要的作用。最近,我们探索了一种针对 E3 连接酶 DCAF11 的烯基吲哚弹头,并试图验证其潜力。在这项研究中,我们合成了一系列具有修饰烯基氧化吲哚弹头的 BRD4 PROTACs(8a-8o、14a-14f、22a-22m),并开发了一种基于高含量成像的高通量筛选系统。我们发现 L134(22a)是一种强效的 BRD4 降解剂,能实现 BRD4 降解(Dmax > 98%,DC50 = 7.36 nM)并显示出抗肿瘤活性。从机理上讲,L134通过泛素-蛋白酶体系统以DCAF11依赖的方式介导BRD4降解。因此,本研究提供了一种快速筛选有效 PROTACs 的方法,并强调基于烯基氧化吲哚-DCAF11 对的 PROTAC L134 是治疗 BRD4 驱动的癌症的一种有希望的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
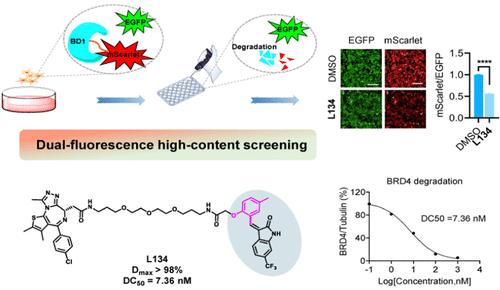
Design, Synthesis, and Activity Evaluation of BRD4 PROTAC Based on Alkenyl Oxindole-DCAF11 Pair
Proteolytic targeting chimera (PROTAC) represent an advanced strategy for targeting undruggable proteins, and the molecular warheads targeting E3 ligases play a crucial role. Recently, we explored an alkenyl oxindole warhead targeting the E3 ligase DCAF11 and sought to validate its potential. In this study, we synthesized a range of BRD4 PROTACs (8a–8o, 14a–14f, 22a–22m) with modified alkenyl oxindole warheads and developed a high-throughput screening system based on high-content imaging. We identified L134 (22a) as a potent BRD4 degrader, achieving BRD4 degradation (Dmax > 98%, DC50 = 7.36 nM) and demonstrating antitumor activity. Mechanically, BRD4 degradation by L134 was mediated through the ubiquitin-proteasome system in a DCAF11-dependent manner. Therefore, this study provides a rapid screening method for effective PROTACs and highlights the PROTAC L134 based on alkenyl oxindole-DCAF11 pair as a promising candidate for treating BRD4-driven cancers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


