双模式活性氧刺激一氧化碳释放,实现光动力和气体肿瘤协同治疗
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
由光产生的活性氧(ROS)模拟的可控一氧化碳(CO)释放是一种很有前景的癌症治疗方法,但传统光敏剂在缺氧肿瘤微环境中的低CO释放率和低ROS生成阻碍了这种方法的发展。在这项研究中,我们通过共封装有机 AIE 光敏剂(PSs)和 CO 原药(Fe3(CO)12),开发了一种高效的纳米平台(TPyNO2-FeCO NPs),它能够在光的触发下产生强大的 ROS 并释放 CO,从而实现协同光动力疗法(PDT)和 CO 气体疗法。通过引入α-光诱导电子传递(α-PET)过程,该纳米平台成功地利用了具有卓越的I型和II型ROS生成能力的PS(TPyNO2)设计。由于加入了 4-硝基苄基单元作为典型的 PET 供体,分子内 α-PET 过程不仅抑制了辐射衰变,将激发态能量重新导向系统间交叉,以形成更多的三态,而且还促进了电子分离和转移过程,从而产生自由基型 ROS。由此产生的 TPyNO2 在聚合态下具有卓越的单线态氧、超氧阴离子和羟基径向生成能力。在光照射下,TPyNO2-FeCO NPs 通过 I 型和 II 型双模式 ROS 介导的过程,以可控和有针对性的方式释放 CO,克服了传统 CO 释放系统的局限性。TPyNO2-FeCO NPs 还展示了一个自加速 ROS-CO-ROS 循环,因为释放的 CO 会诱导细胞内氧化应激,使线粒体膜电位去极化,并抑制 ATP 的产生,从而进一步导致细胞内 ROS 的产生。体外和体内实验均验证了 PDT 和 CO 气体联合疗法的卓越抗肿瘤性能。这项研究为开发先进的 PSs 提供了宝贵的见解,并将 TPyNO2-FeCO NPs 确立为安全有效的抗肿瘤应用前景广阔的纳米平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
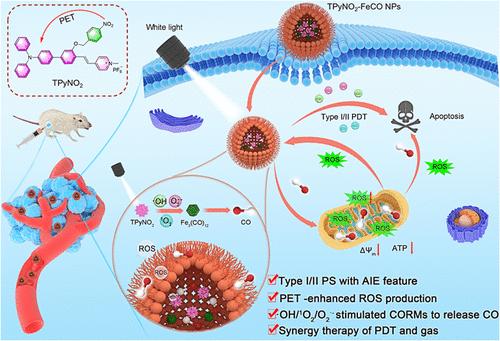
Dual-Mode Reactive Oxygen Species-Stimulated Carbon Monoxide Release for Synergistic Photodynamic and Gas Tumor Therapy
Controllable carbon monoxide (CO) release simulated by light-generated reactive oxygen species (ROS) represents a promising approach for cancer therapy but is hampered by low CO release rate and low ROS generation of conventional photosensitizers in hypoxia tumor microenvironments. In this study, we developed a highly efficient nanoplatform (TPyNO2–FeCO NPs) through co-encapsulating organic AIE photosensitizers (PSs) and CO prodrug (Fe3(CO)12), which are capable of light-triggered robust ROS generation and CO release for synergistic photodynamic therapy (PDT) and CO gas therapy. The success of this nanoplatform leverages the design of a PS, TPyNO2, with exceptional type I and type II ROS generation capabilities, achieved through the introduction of the α-photoinduced electron transfer (α-PET) process. With the incorporation of a 4-nitrobenzyl unit as a typical PET donor, the intramolecular α-PET process not only suppresses the radiative decay to redirect the excited-state energy to intersystem crossing for more triplet-state formation but also promotes electron separation and transfer processes for radical-type ROS generation. The resultant TPyNO2 demonstrates superior singlet oxygen, superoxide anion, and hydroxyl radial generation capabilities in the aggregate state. Upon light irradiation, TPyNO2–FeCO NPs release CO via the type I and type II dual-mode ROS-mediated processes in a controlled and targeted manner, overcoming the limitations of conventional CO release systems. TPyNO2–FeCO NPs also demonstrate a self-accelerating ROS–CO–ROS loop as the released CO induces intracellular oxidative stress, depolarizes mitochondria membrane potentials, and inhibits ATP production, leading to further intracellular ROS generation. Both in vitro and in vivo experiments validated the excellent antitumor performance of the combined PDT and CO gas therapy. This study provides valuable insights into the development of advanced PSs and establishes TPyNO2–FeCO NPs as promising nanoplatforms for safe and effective antitumor applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


