溶解有机物对卤素自由基(Cl2-- 和 Br2--)诱导的微污染物降解的逆还原效应
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
反应性卤素自由基(如 Cl2 和 Br2)对天然水体和工程水处理系统中的微污染物降解有很大影响。众所周知,实际水体中无处不在的溶解性有机物(DOM)会通过减少微污染物的中间产物(即 TC-+/TC(-H)-)来极大地抑制微污染物的降解,但这种 DOM 对卤素自由基诱导系统的影响尚未得到了解。本研究的重点是研究和量化 DOM 在 Cl2---和 Br2---介导过程中的抑制作用。研究选择鸟苷(Gs)作为模型化合物。瞬态光谱显示,Cl2---和 Br2---通过单电子转移与 Gs 反应生成中间产物(即 Gs-+/Gs(-H)-)。在 1.0 mgC L-1 DOM 的存在下,超过 70% 的氧化 Gs 被还原成 Gs。比较不同反应体系的反还原抑制程度,Br2--体系的抑制程度略低于Cl2--和SO4--体系,相应的抑制因子(IF)值也略有不同,分别为SO4-- < Cl2- < Br2--。对 19 种常见微污染物的 DOM 反还原效应进行了进一步量化。在 Cl2---和 Br2---介导的过程中,IF 值分别为 0.21-1.26 和 0.28-1.40,差异很大。在这两个系统中,嘌呤和胺的抑制作用通常比酚类更明显。观察到 IF 值与微污染物的还原电位有很好的相关性,可用于预测更多未经研究的微污染物的降解。这项研究强调了 DOM 的反向还原效应对微污染物降解的重要作用。它可以大大提高在处理含卤化物水的高级氧化过程中预测降解率的准确性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
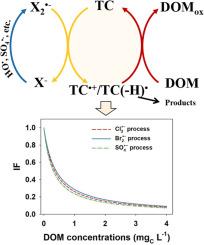
The reverse-reduction effect of dissolved organic matter on the degradation of micropollutants induced by halogen radicals (Cl2•- and Br2•-)
Reactive halogen radicals (e.g., Cl2•- and Br2•-) greatly impact the degradation of micropollutants in natural waters and engineered water treatment systems. The ubiquitous dissolved organic matter (DOM) in real waters is known to greatly inhibit the degradation of micropollutants by reducing micropollutant's intermediate (i.e., TC•+/TC(-H)•), however, such DOM's effects on the halogen-radical-induced system have not been understood yet. The present study focuses on investigating and quantifying such inhibitory effects of DOM during Cl2•-- and Br2•--mediated process. Guanosine (Gs) was selected as a model compound. The transient spectra show that Cl2•- and Br2•- react with Gs generating intermediates (i.e., Gs•+/Gs(-H)•) via single-electron transfer. In the presence of 1.0 mgC L-1 DOM, over 70% of this oxidized Gs was reduced back to Gs. Comparing the extent of reverse-reduction inhibitory among different reaction systems, this inhibitory in Br2•- system was slightly lower than that in Cl2•- and SO4•- system, corresponding the slightly difference of inhibition factor (IF) values as SO4•- < Cl2•- < Br2•-. The reverse-reduction effect of DOM was further quantified for 19 common micropollutants. It varied significantly with IF values of 0.21–1.26 and 0.28–1.40 in Cl2•-- and Br2•--mediated process, respectively. Purines and amines generally exhibited more pronounced inhibition than phenols in both systems. A good correlation of IF values with micropollutant's reduction potential was observed, which can be applied to predict the degradation of more unstudied micropollutants. This study highlights the important role of the reverse-reduction effect of DOM on micropollutant degradation. It can significantly improve the accuracy in predicting degradation rate in advanced oxidation processes for treating water containing halides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


