通过离子调节增强亲水性 TiO2 纳米粒子在气液界面的吸附能力
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
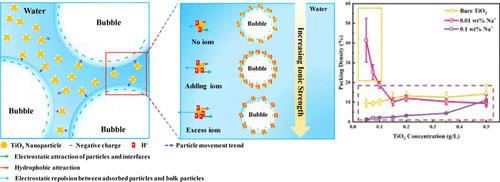
本研究利用垂滴法研究了亲水性 TiO2 P25 的气液界面张力动态特性。此外,研究还考察了亲水性 TiO2 粒子的界面吸附机理,考虑了粒子表面电荷分布特征与离子调节以增强粒子界面吸附的关系。实验结果表明,在不添加离子的情况下,TiO2 P25 悬浮体系因其超亲水性而表现出有限的界面吸附,与颗粒浓度无关。NaCl 的加入增加了颗粒的表面电荷密度,加强了颗粒与界面之间的静电吸引力,增强了颗粒的吸附性。具体来说,在氯化钠浓度较低(0.01 wt %)时,颗粒表面电荷密度和接触角的增加会提高颗粒活性和界面堆积密度。氯化钠浓度较高(0.1 wt %)时,虽然氯化钠会进一步增大颗粒接触角,但由于单个颗粒所占空气-液体界面的有效横截面积增大,导致表面自由能降低。尽管静电吸引力增强,但这导致了堆积密度降低。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Toward an Enhanced Hydrophilic TiO2 Nanoparticles Adsorption at Air–Liquid Interface Through Ion Regulation
This study investigates the dynamic properties of air–liquid interfacial tension for hydrophilic TiO2 P25 utilizing the pendant drop method. Additionally, it examines the interfacial adsorption mechanism of hydrophilic TiO2 particles, considering the characteristics of particle surface charge distribution in relation to ion regulation to enhance particle interface adsorption. Experimental results reveal that in the absence of ion addition, the TiO2 P25 suspension system exhibits limited interfacial adsorption due to its superhydrophilicity, regardless of particle concentration. The addition of NaCl increases the surface charge density of the particles, strengthens the electrostatic attraction between particles and the interface, and enhances particle adsorption. Specifically, at a low NaCl concentration (0.01 wt %), the increased surface charge density and contact angle of the particles elevate particle activity and high interfacial packing density. At a higher NaCl concentration (0.1 wt %), while NaCl further increases the particle contact angle, the increased effective cross-sectional area of the air–liquid interface occupied by individual particles leads to a reduction in surface free energy. Despite the enhanced electrostatic attraction, this results in a lower packing density.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


