BiVO4:Zr,Mo/Pt 薄膜上的甲醇、乙二醇和甘油光电化学氧化反应:比较研究
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
绿色 H2 生产在能源转型中发挥着关键作用,但仍需改进才能大规模应用。水氧化反应作为一个为 H2 生产提供质子和电子的阳极过程,具有动力学上的局限性。因此,在 H2 生产过程中,使用生物质衍生物氧化等替代工艺来取代水氧化反应是非常有必要的。在这项工作中,我们比较研究了不同的小有机分子(甲醇、乙二醇 (EG) 和甘油)在中性介质中原始 BiVO4、掺杂 Mo-Zr 的 BiVO4 和铂辅助催化剂 BiVO4 光阳极上作为水氧化的替代物的情况。虽然不同材料的能带图和表面形态相似,但 Zr-Mo 的存在增加了电荷载流子密度。铂的存在对有机分子起到了辅助催化剂的作用。无论采用哪种材料,在 1.23 V 与 RHE 的线性电位扫频和计时器中,每种有机分子反应的活性顺序为:jwater < jmethanol < jEG < jglycerol。Zr-Mo 和铂的存在提高了醇氧化的光活性,但铂的存在对甘油氧化比对其他物质更有利,在电解 24 小时后仍能保持 80% 的活性。醇的反应活性可通过不同大小的自由基的形成得到初步解释。本文章由计算机程序翻译,如有差异,请以英文原文为准。
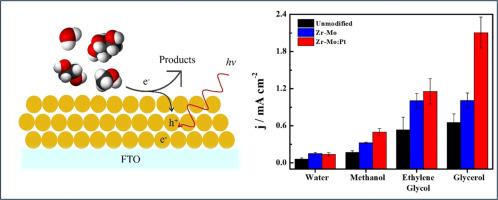
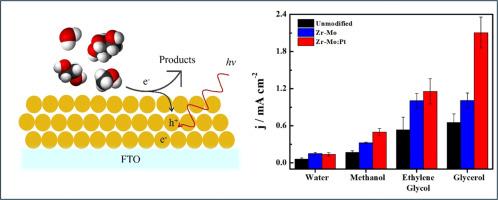
Methanol, ethylene glycol, and glycerol photoelectrochemical oxidation reactions on BiVO4: Zr,Mo/Pt thin films: A comparative study
The green H2 production plays a key role in the energy transition, however it still needs improvements to be applied in a large scale. The water oxidation reaction, as an anodic process to provide both protons and electrons for H2 production, possesses kinetic limitations. Therefore, the use of an alternative process like the oxidation of biomass-derivative species is extremely desired to replace water oxidation during H2 production. In this work, we provide a comparative study of different small organic molecules (methanol, ethylene glycol (EG), and glycerol) as alternatives to water oxidation at pristine BiVO4, Mo-Zr dopped BiVO4, and Pt co-catalyst BiVO4 photoanodes in neutral media. While the band energy diagram and surface morphology are similar for the distinct materials, the presence of Zr-Mo increases the charge carriers density. The presence of Pt acts as co-catalyst for the organic molecules. Regardless of the material employed, the activity order for each organic molecule reaction was: jwater < jmethanol < jEG < jglycerol affeered by both linear potential sweep and chronoamperometry at 1.23 V vs RHE. The presence of both Zr-Mo and Pt increases the photoactivity for the alcohol's oxidation, however the presence of Pt is more advantageous for glycerol oxidation than for other species, maintaining 80 % of activity after 24 h electrolysis. The reactivity of alcohols can be tentatively explained by the greater stability of radicals formed from larger molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


