抗 PD-1 治疗期间肠道微生物群的纵向分析揭示了黑色素瘤患者反应的稳定微生物特征
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
摘要
免疫检查点抑制剂(ICIs)可改善晚期黑色素瘤的治疗效果,但许多患者会出现难治性或复发。肠道微生物群调节抗肿瘤反应。然而,不一致的基线预测因素导致了反应的异质性和横断面数据的不足。我们对无法切除的黑色素瘤患者进行了基线和抗PD-1治疗期间的随访,收集粪便和血液样本,调查肠道微生物群和免疫标记物的变化。在 ICI 治疗期间,患者不同的反应与不同的肠道微生物群动态有关。我们通过稳定的微生物群功能筛选出完全应答者,并通过多个外部队列和实验进行验证。我们确定了主要组织相容性复合体 I 类(MHC I 类)限制肽,这些肽来源于拉赫诺斯皮拉科(Lachnospiraceae)鞭毛蛋白相关基因(FLach),是肿瘤相关抗原的结构同源物,在 ICI 治疗前检测到完全应答者中的 FLach 反应性 CD8+ T 细胞,并证明 FLach 肽能提高抗肿瘤免疫力。这些发现凸显了微生物功能的预后价值和仿肿瘤微生物肽的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
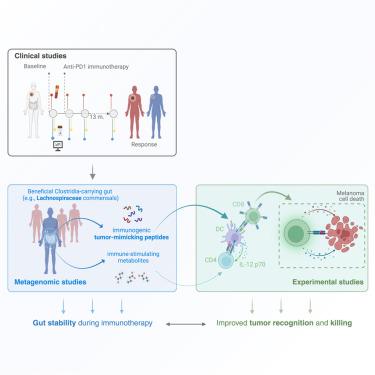
Longitudinal analysis of the gut microbiota during anti-PD-1 therapy reveals stable microbial features of response in melanoma patients
Immune checkpoint inhibitors (ICIs) improve outcomes in advanced melanoma, but many patients are refractory or experience relapse. The gut microbiota modulates antitumor responses. However, inconsistent baseline predictors point to heterogeneity in responses and inadequacy of cross-sectional data. We followed patients with unresectable melanoma from baseline and during anti-PD-1 therapy, collecting fecal and blood samples that were surveyed for changes in the gut microbiota and immune markers. Varying patient responses were linked to different gut microbiota dynamics during ICI treatment. We select complete responders by their stable microbiota functions and validate them using multiple external cohorts and experimentally. We identify major histocompatibility complex class I (MHC class I)-restricted peptides derived from flagellin-related genes of Lachnospiraceae (FLach) as structural homologs of tumor-associated antigens, detect FLach-reactive CD8+ T cells in complete responders before ICI therapy, and demonstrate that FLach peptides improve antitumor immunity. These findings highlight the prognostic value of microbial functions and therapeutic potential of tumor-mimicking microbial peptides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


