作为 Mcl-1 抑制剂的 7-氮杂吲哚-酰磺酰胺衍生物的合成、生物学评价和分子对接研究
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
研究人员设计、合成了一系列7-氮杂吲哚-酰磺酰胺衍生物,并通过荧光偏振试验(FPA)评估了它们对Mcl-1蛋白的抑制活性。其中,最有效的化合物对 Mcl-1 的抑制活性最好,在 50 μM 浓度下的抑制率为 97%,比阳性对照 WL-276 的抑制率(95%)略高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
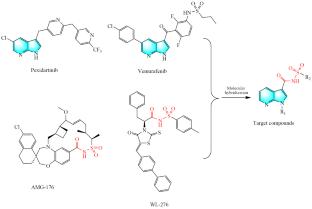
Synthesis, Biological Evaluation, and Molecular Docking Studies of 7-Azaindole-Acylsulfonamide Derivatives as Mcl-1 Inhibitors
A series of 7-azaindole-acylsulfonamide derivatives was designed, synthesized and evaluated for inhibitory activity against Mcl-1 protein through fluorescence polarization assays (FPAs). Among them, the most potent compounds showed the best inhibitory activities against Mcl-1 with the inhibitory rate of 97% at the concentration of 50 μM, which was a little more potent than the positive control WL-276 (95%).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
22.20%
发文量
252
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of General Chemistry is a journal that covers many problems that are of general interest to the whole community of chemists. The journal is the successor to Russia’s first chemical journal, Zhurnal Russkogo Khimicheskogo Obshchestva (Journal of the Russian Chemical Society ) founded in 1869 to cover all aspects of chemistry. Now the journal is focused on the interdisciplinary areas of chemistry (organometallics, organometalloids, organoinorganic complexes, mechanochemistry, nanochemistry, etc.), new achievements and long-term results in the field. The journal publishes reviews, current scientific papers, letters to the editor, and discussion papers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


