探索根据生物环境选择钼多酶和钨多酶背后的自然鉴别因素
IF 20.3
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
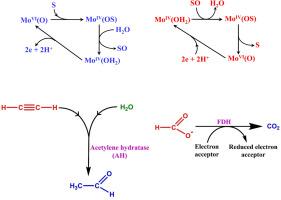
钼依赖酶中的蝶呤钼辅助因子可催化氧化转移酶和羟化酶的活性。就钨而言,基于蝶呤的钨辅助因子在嗜热微生物的厌氧条件下具有类似的生物低电位氧化还原活动。大自然根据钨和钼的相对生物利用率、其化合物的热力学稳定性、动力学惰性以及这两种同系物在相对论效应方面的差异,合理地选择了钨和钼用于不同的工作条件。相对论效应是证明钨与钼在不同酶中的选择性的最重要因素。基于蝶呤配体的非无辜二硫环戊烯通过稳定钼和钨的 +4、+5 和 +6 氧化态来调整酶的生物氧化还原活性。事实上,它在酶的催化机制中起着 "氧化还原缓冲器 "的作用。与依赖钼的酶相比,依赖钨的酶的酶活性机理研究更多。关于 Mo/W-FDH(甲酸脱氢酶)、Mo-Cu-CODH(一氧化碳脱氢酶)、W-AOR(醛氧化还原酶)、W-AH(乙炔氢化酶)、W-BCRs(苯甲酰-CoA 还原酶)等酶的活动机制的激烈争论表明,这一领域仍然是一个活跃的研究领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the nature’s discriminating factors behind the selection of molybdoenzymes and tungstoenzymes depending on the biological environment
Pterin based molybdenum cofactor found in the molybdenum dependent enzymes catalyzes the oxo-transferase and hydroxylase activity. For tungsten, the pterin based tungsten cofactor is known for the similar biological low potential redox activities in anaerobic conditions in thermophilic microorganisms. Nature's selection of tungsten and molybdenum for the different working conditions is rationalized in terms of their relative bioavailabilities, thermodynamic stabilities of their compounds, kinetic inertness and the difference in relativistic effect experienced by these two congeners. The relativistic effect is the most important factor to justify the tungsten vs. molybdenum selectivity in different enzymes. The non-innocent dithiolene based pterin ligand tunes the biological redox activity of the enzymes by stabilising +4, +5 and +6 oxidation states of molybdenum and tungsten. In fact, it acts as a ‘redox buffer’ in their catalytic mechanism. Mechanistic aspects of the enzymatic activity are more investigated for the Mo-dependent enzymes compared to those of W-dependent enzymes. Strong controversies regarding the mechanisms of activity of the enzymes like Mo/W-FDH (formate dehydrogenase), Mo-Cu-CODH (carbon monoxide dehydrogenase), W-AOR (aldehyde oxidoreductase), W-AH (acetylene hydratase), W-BCRs (benzoyl-CoA-reductases), etc., indicate that this field is still an active area of research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Coordination Chemistry Reviews
化学-无机化学与核化学
CiteScore
34.30
自引率
5.30%
发文量
457
审稿时长
54 days
期刊介绍:
Coordination Chemistry Reviews offers rapid publication of review articles on current and significant topics in coordination chemistry, encompassing organometallic, supramolecular, theoretical, and bioinorganic chemistry. It also covers catalysis, materials chemistry, and metal-organic frameworks from a coordination chemistry perspective. Reviews summarize recent developments or discuss specific techniques, welcoming contributions from both established and emerging researchers.
The journal releases special issues on timely subjects, including those featuring contributions from specific regions or conferences. Occasional full-length book articles are also featured. Additionally, special volumes cover annual reviews of main group chemistry, transition metal group chemistry, and organometallic chemistry. These comprehensive reviews are vital resources for those engaged in coordination chemistry, further establishing Coordination Chemistry Reviews as a hub for insightful surveys in inorganic and physical inorganic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


