KNb0.99Eu0.01O3 的合成、表征以及 Ag/La2NiO4/ KNb0.99Eu0.01O3 异质结的光催化 H2 产率改进
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-10-15
DOI:10.1016/j.jphotochem.2024.116094
引用次数: 0
摘要
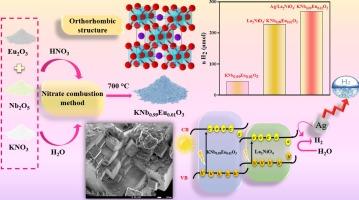
通过硝酸盐燃烧制备的过氧化物 KNb0.99Eu0.01O3 与 La2NiO4 异质结在可见光下用作氢气光电阴极。XRD 图谱证实了 KNb0.99Eu0.01O3 结构为正交单相。扫描电子显微镜图像显示出 0.2 至 0.4 微米之间的晶粒,而光带隙(3.14 eV)是通过漫反射计算得出的。在照明条件下,Na2SO4 电解液中的循环伏安法(CV)和电容测量结果表明,相对于 Ag/AgCl 而言,电流在 ∼ -0.7 V 以下时会增大,并且具有 p 型行为,导带(CB = -1.95 V)相对于 H2 水平阴极定位,从而导致 H2 光生。异质系统 La2NiO4/KNb0.99Eu0.01O3 的活性远高于 KNb0.99Eu0.01O3,在异质系统中,H2-光解与作为还原剂的 S2O32- 的氧化同时发生。因此,La2NiO4 与 KNb0.99Eu0.01O3 的共结合促进了电荷转移,提高了反应效率。在中性 pH 值下,KNb0.99Eu0.01O3 和 La2NiO4/ KNb0.99Eu0.01O3 光催化剂的 H2 演化率分别为 11.15 和 57 µmol g-1 min-1。Ag 负载的 La2NiO4/KNb0.99Eu0.01O3 的产氢率进一步提高,达到 67 µmol g-1 min-1 的最大产氢率,这归功于 Ag 带来的较低过电位。这些发现凸显了 Ag/La2NiO /KNb0.99Eu0.01O3 三元体系作为高效光阴极产生氢气的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis, Characterization of KNb0.99Eu0.01O3 and improved Photo-catalytic H2-Production on Ag/La2NiO4/ KNb0.99Eu0.01O3 hetero-junction
The perovskite KNb0.99Eu0.01O3 prepared by nitrate combustion is applied as a hydrogen photocathode in heterojunction with La2NiO4 under visible light. The XRD pattern confirms the formation of KNb0.99Eu0.01O3 structure with orthorhombic single phase. The SEM images present grains between 0.2 and 0.4 µm while the optical band gap (3.14 eV) is calculated from the diffuse reflectance. Under illumination, the cyclic voltammetry (CV) and the capacitance measurements in Na2SO4 electrolyte show an increased current below ∼ -0.7 V vs. Ag/AgCl and p-type behavior with a conduction band (CB = −1.95 V) cathodically positioned with respect to H2 level, thus leading to H2 photo-production. The hetero-system La2NiO4/KNb0.99Eu0.01O3 shows a much higher activity than KNb0.99Eu0.01O3 where efficient H2-photoevolution occurs with concomitant oxidation of S2O32−, as reducing agent. So, the co-junction of La2NiO4 with KNb0.99Eu0.01O3 facilitates the charge transfer and increases the reaction efficiency. At neutral pH, the H2 evolution rate for the photocatalysts KNb0.99Eu0.01O3 and La2NiO4/ KNb0.99Eu0.01O3 are 11.15 and 57 µmol g−1 min−1; respectively. Further enhancement is observed with Ag-loaded La2NiO4/KNb0.99Eu0.01O3, reaching a maximum hydrogen production rate of 67 µmol g−1 min−1, attributed to the lower overpotential introduced by Ag. These findings highlight the potential of the ternary system Ag/La2NiO /KNb0.99Eu0.01O3 as an efficient photocathode for H2 generation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


