一种氮杂环戊烯在 1,2-二氯乙烷中的光化学质子化反应
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-10-20
DOI:10.1016/j.jphotochem.2024.116106
引用次数: 0
摘要
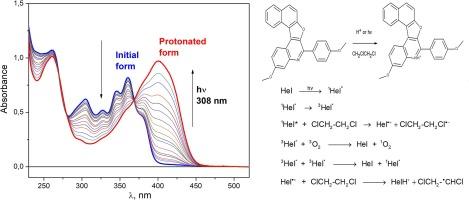
菲啶杂环类似物是一类重要的含氮化合物,具有良好的光学特性,包括高发光量子产率、高摩尔吸收系数以及对溶剂介质的敏感性。通过在菲啶框架的不同位置引入适当的取代基,可以改变菲啶及其衍生物的发光特性。最近开发的呋喃喹啉系列发光氮杂环戊烯在吸收光谱和发射光谱上都表现出强烈的酸致变色效应。这些化合物的特点之一是在紫外线照射下,在含氯溶剂中通过光化学作用形成质子化形式。在这项工作中,我们通过固定和激光闪烁光解的方法,对 1,2 二氯乙烷(1,2-DCE)中的 3-甲氧基-6-(4-甲氧基苯基)萘并[1′,2′:4,5]呋喃并[2,3-c]喹啉(化合物 1)进行了机理研究。除了在典型有机溶剂中观察到的三重态形成外,还发现在 1,2-DCE 中光激发会导致电子从激发的化合物 1 转移到溶剂分子。由此产生的中间产物发生反应,形成质子化的氮杂环戊烯。提出了光质子化的定量机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Photochemical protonation of an azahelicene in 1,2-dichloroethane
Heterocyclic analogues of phenanthridine are an important class of nitrogen-containing compounds with promising optical properties including high quantum yield of luminescence, high molar absorption coefficients and sensitivity to solvent medium. The luminescent properties of phenanthridine and its derivatives can be changed by introducing appropriate substituents into various positions of the phenanthridine framework. Recently developed luminescent azahelicenes of furoquinoline series demonstrate strong acidochromic effect both on absorption and emission spectra. One of the features of these compounds is the photochemical formation of a protonated form in chlorine-containing solvents under UV irradiation. In this work we perform a mechanistic study of 3-methoxy-6-(4-methoxyphenyl)naphtho[1′,2′:4,5]furo[2,3-c]quinoline (compound 1) in 1,2-dichloroethane (1,2-DCE) by means of stationary and laser flash photolysis. In addition to the triplet state formation observed in typical organic solvents photoexcitation in 1,2-DCE was found to result in an electron transfer from the excited compound 1 to a solvent molecule. Reactions of resulting intermediates lead to the formation of the protonated azahelicene. The quantitative mechanism of photoprotonation is put forward.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


