具有 ESIPT 功能的嘧啶配体基底上的配位驱动分子开关:合成、卤化物阴离子对发射的微调和理论研究
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-10-19
DOI:10.1016/j.jphotochem.2024.116091
引用次数: 0
摘要
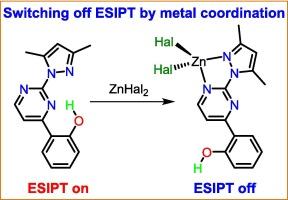
基于 ESIPT 的材料(ESIPT = 受激态分子内质子转移)由于其发光特性的特殊性,在光电子学和生物医学领域有着广泛的应用。本文合成了一种具有 ESIPT 功能的化合物 2-(3,5-二甲基-1H-吡唑-1-基)-4-(2-羟基苯基)嘧啶(HL4,2,Me),该化合物具有一个短的 O-H⋅⋅N 分子内氢键和两个 N,N 位点用于金属结合。HL4,2,Me是第一个报道的无需去质子化就能通过金属离子配位触发ESIPT分子开关的分子。与游离配体相比,[Zn(HL4,2,Me)X2](X = Cl、Br、I)复合物中 HL4,2,Me 构象的急剧变化极大地改变了光致发光响应。在固态下,HL4,2,Me 表现出无障 ESIPT 和大的斯托克斯偏移黄橙色发射,这是由于反卡沙 S2 → S0 荧光和类似卡沙的 T1 → S0 磷光辐射通道相互作用的结果。HL4,2,Me违反卡沙规则的原因是S2-S1能隙(约 0.9 eV)过大,减缓了S2 → S1内部转换的速度。ESIPT 抑制的锌(II)配位化合物的光致发光行为在很大程度上取决于卤化物阴离子:氯代配合物只表现出荧光,溴代配合物除了主要的荧光通道外还表现出次要的磷光通道,而碘代配合物则主要表现出磷光。因此,[Zn(HL4,2,Me)X2] 复合物的发射颜色从 X = Cl 时的蓝色逐渐变为 X = I 时的橙色,为卤化物阴离子微调发射提供了一个平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Coordination-driven molecular switch on the base of an ESIPT-capable pyrimidine ligand: Synthesis, fine-tuning of emission by the halide anion and theoretical studies
ESIPT-based materials (ESIPT = Excited State Intramolecular Proton Transfer) find diverse applications in optoelectronics and biomedicine owing to the peculiarities of their luminescence properties. Here, an ESIPT-capable compound 2-(3,5-dimethyl-1H-pyrazol-1-yl)-4-(2-hydroxyphenyl)pyrimidine (HL4,2,Me) featuring a short O–H⋅⋅⋅N intramolecular hydrogen bond and two N,N-sites for metal binding has been synthesized. HL4,2,Me is the first reported molecule which can act as an ESIPT molecular switch triggered by metal ion coordination without its deprotonation. Drastic changes in the HL4,2,Me conformation in the [Zn(HL4,2,Me)X2] (X = Cl, Br, I) complexes significantly alter the photoluminescence response compared to the free ligand. In the solid state, HL4,2,Me exhibits barrierless ESIPT and large Stokes-shifted yellow-orange emission due to the interplay of anti-Kasha S2 → S0 fluorescence and Kasha-like T1 → S0 phosphorescence radiative channels. The violation of Kasha’s rule for HL4,2,Me is justified by an extraordinarily large S2 – S1 energy gap (ca. 0.9 eV), slowing down the rate of S2 → S1 internal conversion. The photoluminescence behavior of the ESIPT–incapable zinc(II) coordination compounds strongly depends on the halide anion: the chlorido complex exhibits only fluorescence, the bromido complex displays a minor phosphorescence channel in addition to a major fluorescence channel, while the iodido complex exhibits predominantly phosphorescence. As a result, the emission color of the [Zn(HL4,2,Me)X2] complexes changes gradually from blue for X = Cl to orange for X = I, providing a platform for the fine-tuning of emission by the halide anion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


